13 Informing Adaptation with Lessons Learned from Key 21st Century Infectious Disease Outbreaks
Shirley Feldmann-Jensen, DPPD, MPH, RN, PHN and Terrence O’Sullivan, PhD, MAAS
Authors
Shirley Feldmann-Jensen, D.P.P.D., M.P.H., P.H.N., R.N., California State University, Long Beach
Terrence M. O’Sullivan, Ph.D., M.A.A.S., University of New Hampshire
Keywords
pandemic, public health emergencies, infectious disease outbreaks, emerging infectious disease, improvement planning, outbreak governance, lessons learned
Abstract
Infectious diseases (IDs) know no borders, and Covid-19 tragically and dramatically illustrated that widespread globalization-related trade, travel, migration, and human environmental stressors have worsened the ID threat. The world is battle-weary by the COVID-19 pandemic, yet in many regards less ready for “the next one(s)” from the levels of broader government policy and systemic issues to specific measures that are and will need to be taken to mitigate and effectively respond. Global microbial risks grow steadily in both frequency and salience – exacerbated by budgetary cuts to research, surveillance, and preparedness; the deterioration of both public health and medical infrastructures; the global heating crisis; and anti-science (and anti-government) attitudes damaging institutional credibility. The overall effect is an increase in outbreak frequency, which affects global health and just-in-time global supply chains and economies. Thus, it remains more important than ever for both infectious disease disaster-related stakeholders and students of EM to understand how unique and often highly complex ID disasters can be.
This chapter analyzes notable 21st Century case studies and the international dynamics in response to the outbreaks, which provides foundational discussion for ground level risk management. Importantly, the chapter identifies specific areas for affecting more cohesive collective action in preparing for, responding to, and mitigating future outbreaks within and across borders. Although outbreak risk cannot be eliminated, the cumulative lessons learned across the variety of outbreaks of the 21st Century holds value for frontline stakeholders, emergency management students, and wider systems stability.
Introduction
This chapter examines the significance of infectious diseases for emergency management, covering the first quarter of the 21st century. Antecedent factors driving the emergence and spread of infectious diseases in the disaster setting will be covered. In this chapter, representative cases in point will be used to establish a foundation for understanding biological disasters.
Infectious diseases (IDs) know no borders, and Covid-19 tragically and dramatically illustrated that widespread globalization-related trade, travel, migration, and human environmental stressors (such as global heating and natural resource damage) have worsened the ID threat. Those global public health (PH) risks steadily grow in both frequency and salience – exacerbated by budgetary cuts to research, surveillance, and preparedness; the deterioration of both public health and medical infrastructures; the global warming crisis; and anti-science (and anti-government) attitudes damaging institutional credibility.
Understanding how unique and often highly complex ID disasters can be is more important than ever for both infectious disease disaster-related stakeholders and students of emergency management (EM). It might be said that public health is late to disaster risk management, just as the latter is late to the former. The world is battle-weary by the COVID-19 pandemic but in many regards less ready for “the next one(s)”. Effective mitigation and response by all stakeholders is essential from the levels of broader government policy and systemic issues to specific measures.
Outside of the HIV-AIDS pandemic, for many decades international public health experts have warned of growing pandemic or other emergent (novel to humans) and re-emergent (existing but expanding in territory, scope, or severity) disease disaster threats, ranging from pandemic viral flu to mosquito- and other vector-borne pathogens. Relatively robust Western-funded global detection, surveillance, and research infrastructure was in place in the 1960s and 70s, but this along with local, national, and other international public health systems have been significantly cut back/deprioritized in part because of ID/public health complacency.
Dominating public health ID were two main pandemic concerns: 1). a new influenza (or a substantially genetically changed virus) – including “candidate” strains such as H5N1 or H7N9, or 2). a new, previously unknown zoonotic “virus x” emerging from animal hosts – such as pandemic SARS-2 (Covid-19). At the same time, other rising PH disaster threats that do not yet pose pandemic likelihood include:
- Covid’s previous coronavirus cousins, including the original 2003 SARS-CoV and MERS, as well as Ebola, Zika, Dengue fever, West Nile, Nipah, Lyme disease, and other pathogens;
- Biological terrorism, in a context of exploding biotechnology capabilities
- Agents (accidentally or deliberately) released from research laboratories, field experiments, or production facilities
- Rapidly growing dangerous antimicrobial resistance – risking a “post antibiotic era”
- The ability of climate crisis, disasters, and other global trends to greatly exacerbate respiratory, waterborne, and other pathogenic disease risks.
- And the role of “superspreaders” in complicating containment and surveillance.
In addition, current policy challenges include debate about prevention vs. surveillance vs. response priorities; realization that systems cannot stabilize without better integration and collaboration across diverse levels of governance (local to international) – including solving information and communication flow problems across bureaucratic silos; how to make supply chains (for PH and every other thing) more resilient to disruptive ID events; and continuity of business and government (especially personnel shortages).
The purpose of this chapter is to explore the implications of the response and recovery to representative pandemics and outbreaks that have occurred in the 21st century, seeking to answer the question: “What have we learned from 21st century outbreaks that can collectively inform risk management improvement?” The chapter analyzes the historic cases and international dynamics and provides specific discussion of gaps and misalignments affecting cohesive action, system stability, and ground level risk management.
Importantly, the analysis reveals specific areas for taking collective action in preparing for, responding to, and mitigating future outbreaks within and across borders. The growing risk of ID outbreaks interacts with population movements, environmental degradation, a changing climate, and other social factors, illustrating the complexity and ongoing uncertainty of outbreaks. The overall effect is an increase in outbreak frequency, which affects global health, the just in time global supply chain, and economies. Although outbreak risk cannot be eliminated, the cumulative lessons learned across the variety of outbreaks of the 21st century thus far holds value for both frontline stakeholders and emergency management students.
Literature Review
This literature review provides foundational constructs for understanding ID for the emergency management function and reviews the global systems in place for disease control. The significance of infectious disease spread is then examined, along with interacting factors that influence agent transmissibility. Finally, important historical pandemics are reviewed for the context of the societal disruption brought, with an emphasis on influenza, since it remains among the most likely highly disruptive agents.
The control of communicable diseases has been a historical mainstay of public health practice. With the disease fighting tools of modern vaccination, sanitation, vector control practices, and the use of scientific methods in epidemiology, pharmacology, and other areas, the impact of preventable infectious diseases on the developed world markedly decreased during the 20th century. The disability adjusted life years (DALY), a measure of disease burden on those infected by communicable diseases, has been reduced by 50% since 2000 (WHO, 2024), and the global burden of disease caused by infectious agents has been reduced to less than a quarter of worldwide deaths.
Powerful changes occurring across the social, built, and physical environments of the planet are redistributing the consequences of these microbes. This new and sobering frontier includes rapid urbanization, competition for water and food, climate change, environmental degradation, an erosion of global public health infrastructure, and the emergence of novel and drug resistant pathogens (Feldmann-Jensen, 2014). The relationship between these indicators is they each can result in population health emergencies as new infectious diseases appear, and some ancient scourges present modern challenges.
Highlighting basic public health concepts and definitions at the outset helps to establish a common language on which to base subsequent context through the chapter as well as for emergency management practice. Most importantly, the role of public health is centered on health outcomes across the overall population, as well as within defined populations. Public health partners with a community to protect and improve the health of that community, including preventive medicine, sanitary, and social sciences. Notably, this partnership has a concurrent function with emergency management during an infectious disease outbreak. For that reason, specific operational terms used in public health practice during an infectious disease outbreak are delineated and defined in Breakout Box 1.
Breakout Box 1: Public Health Terms and Definitions
Acute – sudden and fast paced way an infection can spread.
Containment – infection control strategies to prevent or stop the spread of infections. (CDC,2024)
Disability-Adjusted Life Year – (DALY) a time-based measure that combines years of life lost due to premature mortality (YLLs) and years of life lost due to time lived in states of less than full health, or years of healthy life lost due to disability (YLDs) (WHO, 2024)
Drug Resistant- antimicrobial resistance happens when bacteria and fungi develop the ability to defeat the drugs designed to kill them. (CDC, 2024)
Emerging & Re-emerging infections (EIDs)– “infections that have newly appeared in a population or have existed previously but are rapidly increasing in incidence or geographic range” (Morens, et al., 2004).
Endemic – a consistently present disease occurrence within a specific geography (CDC, 2024)
Epidemic – an unexpected increase in the number of disease cases in a specific geographic area at a point in time (CDC, 2024).
Epidemiology – the study of the distribution and determinants of health-related states or events in specified population, and the application of this study to the control of health problems (CDC, 2024).
Herd immunity- resistance to the spread of an ID within a population that is based on preexisting immunity as a result of previous infection or vaccination. The key is that there are too few susceptible hosts to maintain transmission (Feldmann-Jensen, 2014).
Incidence rate- A measure of the frequency with which an event, such as a new case of illness, occurs in a population over a period of time. The denominator is the population at risk; the numerator is the number of new cases occurring during a given time period (Feldmann-Jensen, 2014).
Incubation period -the time from exposure to an infectious agent until the time of the fist signs or symptoms of disease (CDC; van Seventer et al., 2017).
Index case -the case first introducing an infectious agent into a setting (Van Seventer, et al., 2017).
Infectious Disease - illness due to a pathogen or toxic product arising through transmission from an infected person, infected animal, or a contaminated inanimate object to a susceptible host (van Seventer et al., 2017).
Infectivity - the likelihood an agent will infect a host (CDC, 2024)
Isolation – separates sick people with a contagious disease from people who are not sick (CDC, 2024).
Morbidity – Any departure from a state of physiological or psychological well-being.
Mortality rate - A measure of the frequency of occurrence of death in a defined population during a specified interval of time.
Outbreak – the occurrence of cases of diseases in excess of what would normally be expected in a defined geography (WHO)
Pandemic – a global outbreak (WHO)
Pathogenicity – the ability of an agent to cause disease if it infects a host (CDC, 2024)
Prevalence - an indicator of the number of existing cases in a population at a given point in time (Van Seventer et al., 2017).
Public health emergency – those events that “adversely impacts the public health system and/or its protective infrastructure, resulting in both direct and indirect consequences to the health of a population, and occur when this protective threshold is absent, destroyed, overwhelmed, not recovered, or maintained, or denied to populations” (Burkle, 2008).
Quarantine- separates and restricts the movement of people who were exposed to a contagious disease to see if they become sick (CDC, 2024).
Reservoir - one or more epidemiologically connected populations or environments in which a pathogen can be permanently maintained and from which infection is transmitted to the defined target species (Cutler et al., 2010).
Surge capability – the ability to address unusual or very specialized medical needs (ASPR, HHS, 2024).
Surge capacity–the ability to respond to a markedly increased number of patients (ASPR, HHS, 2024).
Vector born disease – Human illnesses caused by parasites, viruses, and bacteria that are transmitted by vectors such as, mosquitos, ticks, and fleas. (Boischio et al., 2009)
Virulence -structural or biochemical properties of an infectious agent that affect the likelihood of causing severe disease among those with disease (van Seventer et al., 2017).
Zoonotic transmission – Zoonoses are infectious diseases that spread between animals and humans. (CDC, 2024)
Infectious Disease
Infectious diseases (ID) have been a part of the world for centuries and were both commonplace and disastrous before the first antibiotics were developed (Garrett, 2005). Occurrences of catastrophic epidemics and pandemics in past eras were great regulators of population and mediated social systems (Bray, 2000).
Today, the ID burden is far greater among developing nations and regions with stark disparities (Quinn et al., 2010; Gray et al., 2022), where the higher morbidity and mortality is related to social, demographic, and environmental factors such as migration and unplanned urbanization. These factors ultimately limit access to preventive health, vaccines, medicines, and supportive clinical medical care. Another dimension is the growing burden of non-communicable disease world-wide, where concurrent infections become both causal and intermediate sources of death (Gray et al., 2022). To illustrate this dynamic further, 13.7 million deaths were attributed to infections in 2019, with 5.2 million of those deaths occurring in combination with chronic disorders (Gray et al., 2022). This interaction of infectious disease with other environmental factors is explained by theory, including the Epidemiological Triangle.
Epidemiological Triangle
The Epidemiological Triangle is the enduring model which explains infectious disease causality. This pattern depicts a system of connections and interdependencies that determine the degree to which a microbe will cause illness in the human population. The outcomes of the interactions include the properties of the infectious agent, environmental factors, and characteristics of the host. For example, when a likely host is exposed to an infectious agent, the outcome of that exposure varies with the agent factors and the host factors in the interaction, while the environment factors are external determinants of a host vulnerability (van Seventeer et al., 2017). Figure 1 below, is an illustration of this model, which depicts the characteristics of agents, hosts, and the environment.
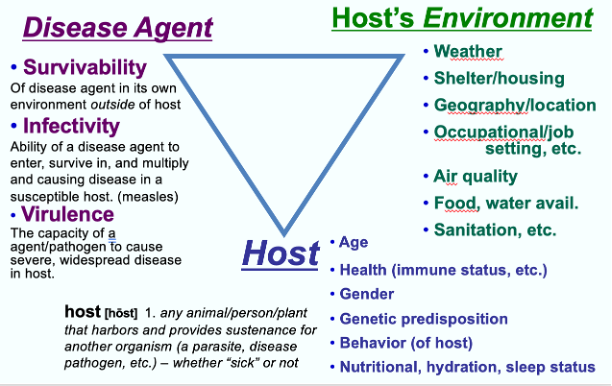
“Agent” characteristics determine the likelihood of propagating infection. These attributes include survivability of its own environment outside the host; infectivity, the likelihood it will infect a host; pathogenicity, the ability to cause disease; and virulence, the property determining the severity of disease (van Seventeer, 2017).
“Host” characteristics are qualities that will affect the organism's susceptibility to both infection and disease. Genetic or acquired factors, age, gender, stress, pregnancy, nutritional status, and chronic diseases can all provide either strengths or weaknesses for preventing infection or severe disease (van Seventeer, 2017).
“Environment” characteristics can influence the host’s risk of exposure as well as vulnerability to infection. These determinants can have profound impact on disease outcomes as well, and include “physical, social, behavioral, cultural, political, and economic systems” (van Seventeer, 2017). Because of the substantive environmental and human effects on ID outcomes, it is worth discussing these interactive factors in further detail.
Influencing Human and Environmental Factors
As can be seen, many complexities and interactions determine causality. The circumstances in which people are born, grow up, live, work, and age, and the systems put in place to deal with illness have been substantiated by the World Health Organization (WHO) to affect health outcomes. These circumstances are in turn shaped by a wider set of forces: economics, social practices, and politics and government policies. The structural conditions form what is now commonly referred to as the social determinants of health. Notably, a similar set of forces shape the social determinants of risk in the all-hazards environment. Therefore, a deeper look into literature surrounding the social and environmental determinants and their effect upon the microbe and human connection is essential. This includes demographic changes, urbanization, globalization, environmental degradation and climate change, incursion into biodiverse zones, and socioeconomic inequality among other things.
Demographic Changes & Movement
The migration or movement of people is age-old, complex, and influenced by the environmental changes, civil strife, and economic/resource conditions. “Ideas, information & people follow armies and economic flows and in so doing transform societies” (Nye, J.S., and Donahue, J.D., 2000). The push-pull factors that stimulate migration include movement from unsafe to safe, poor to rich, or rural to urban movement (Migration Policy and Research Program, 2005). Population movements widen the spread of microbes, degrade environments, further disease transmission, increase vector habitat range and give rise to new infectious strains (Feldmann-Jensen, 2014).
Urbanization
Modern metropolitan regions are migration and growth magnets. Historically, however, before modern sanitation and public health, instead of a better life, higher risks to health and wellbeing were often found in these dynamic environments.
The growth of urban areas is influenced by population growth as well as rural to urban migration (Montgomery, 2010), with the lack of investment in rural services being another driver. In developing nations, urban growth has outpaced the abilities of governments to build and maintain essential infrastructures (WHO and UN HABITAT, 2010) and thus, the evolution of informal settlements or slums. The United Nations (UN) estimates also indicate a massive deficit in provision for water and sanitation in these urban areas. Consequently, informal settlements can be characterized by inequality, poverty, and environmental degradation (Feldmann-Jensen, 2014).
Higher urban population densities can generate increased rates of infectious disease transmission, though modern public health infection control can reduce that vulnerability as well (Kendall,C., et al., 1991). Because more than half of the world’s population now lives in urban settings, the effects of population density cannot be overstated.
Globalization
Globalization has been described by Castells as “a multidimensional process to which a given system becomes global to function as a unit in real or chosen time on a planetary scale” (2011, speaking notes). An important feature is that these processes benefit those inside the network of globalization, but there are many who remain outside the network. This polarization of inclusion/exclusion contributes to the fragmentation seen within and between societies (Castells, 2009, p. 20). From a public health position, this disparity can be observed in access to and distribution of health resources and the resulting health outcomes throughout societies.
The relationship of globalization to health has been established historically, and it is important to emphasize that the degree of globalization and the spread of infectious disease have followed a parallel course throughout history (Institute of Medicine, 2006). Subsequently, it is helpful to distinguish that population health is considered affected by globalization when the spread of transmissible diseases is spatially wider and temporally faster than it may have been otherwise (Feldmann-Jensen, 2014).
In the current globalized context, no location can be secure from the threat of an emerging infectious disease, even though the outbreak may begin in a seemingly remote part of the world (Quinn, T. & Bartlett, J., 2010). Geographic expansion of infectious diseases can be accelerated by international commercial trade and increased air travel, and spread worldwide through the movements of humans, livestock, insects, food, transportation systems or any combination of these (Feldmann-Jensen, 2014).
Environmental Degradation and Climate Change
The poor stewardship of our planetary health has important human health implications. Environmental degradation and climate change have direct effects on population health, which include aspects related to air quality, food availability, severe weather events, and microbial pattern changes. Environmental degradation is therefore a key factor in the emergence or re-emergence of some infectious diseases.
Climate Change entails more than just rising average temperatures. The evidence in long term changes includes extreme weather components (heat waves and increasing intensity of tropical cyclones and typhoons), varying precipitation amounts (drought and heavy rainfall), differences in ocean salinity, altering wind patterns, etc. (IPCC, 2007). These climate-related and other environmental mechanisms both intensify public health challenges and environmental disasters and expand the territory of mosquitos and other vectors, bringing disease to new regions and worsening outbreaks in endemic areas (Boischio et al., 2009).
Incursion into Biodiverse Zones
More than 60% of emerging pathogens originate from zoonotic (animal) diseases (Quinn, T. & Bartlett, J., 2010), and can be often associated with human development and other encroachment into bio-diverse zones, animal migration from habitat degradation and increased contact with wildlife. Some new human pathogens pose greater vulnerability to severe outbreaks because no previous human exposure or herd immunity exists – as is notably the case with novel influenza strains.
Inequality, Poverty, & Civil Unrest
The substantiated importance of the social determinants of health includes the external forces of economic, political, and societal influences. Poverty has been identified as a primary cause of illness in lower-income countries (Clifford, et al., 2008, p. 1). Further, poverty limits people’s access to food and water, ability to use proper disease prevention measures, to secure adequate healthcare, or to act in environmentally responsible ways (Boischio, et al., 2009). Equity and human rights are more than efficiency of delivery in the field of health (Farmer et al., 2005) especially when endeavoring to contain the outcomes of microbial outbreaks.
Evidence is clear that the poor are affected disproportionately because of inequities in basic living conditions, access to health care and migration patterns (Saker, et al., 2004). Infant mortality statistics reveal that “infants in poor and more crowded portions of cities are four times more likely to die” (Quinn, T. & Bartlett, J., 2010). Higher child mortality rates are also evidenced among crowded city slums in contrast to other areas of the same cities (UNHABITAT, 2007). Pathogens and vectors endemic to rural environments are conveyed into urban areas. Poverty, crowding, lack of services, and frequent migration in and around urban areas have all created ideal conditions for propagation of disease (Kendall, C., et al., 1991). As a result, transmission and incidence of infectious disease can also be expected to flourish in these environments.
The variables discussed interact together to determine the social components of risk. As the pathogens flourish, they also get transported into the mobile global society. And so, the “multiplication of risks causes world society to contract into one community of danger” (Beck, 1992, p. 44), as discussed next.
Infectious Disease Outcomes
Commonalities exist among infectious diseases, based upon the disease outcomes. These shared results can be described by three basic characteristics: 1) disability causing, 2) high mortality causing, and 3) emerging/re-emerging (Migration Policy and Research Program, 2005).
“Disability causing infectious diseases” are often endemic in Lesser Developed Countries (LDCs) amid poverty, where people have minimal access to vaccine or medication. Examples of such diseases include dengue fever (profiled below), parasitic worms, other vector borne diseases, diarrheal diseases, hepatitis, cholera, and typhoid. In this disease category, the debilitating effect of illness causes loss of productivity and livelihood, creating even greater vulnerability to the household. These often-neglected diseases affect billions of people, many of whom move into and around cities, helping transmit the infections to mosquitos and other vectors, and other susceptible people (Migration Policy and Research Program, 2005).
“High mortality infectious diseases” in contrast, have higher death rates and can be endemic to specific populations, based on environmental or behavioral variables. Examples include he malaria or HIV pathogens and the populations they affect. Vaccines are generally not available for these agents.
“Emerging and re-emerging infectious diseases” (EID) can range from mild to severe risks, but many possess traits of grave concern for global public health. Contributing factors to the re-emergence of a disease once thought to be under control are antimicrobial resistance, environmental degradation, and global heating causing expansion of insect vector ranges (especially mosquitos) further north or south of the equator. Emerging infectious diseases are new or novel pathogens that humans have not encountered before or have mutated to a new configuration. Newly transformed strains have the potential to find little immunity among large percentages of the world’s population, and thus humanity has the constant threat of another pandemic hanging over its head. The potential disruption produced by a serious emerging infectious disease can be confounding to response and recovery systems. Aside from novel coronaviruses (as the world now recognizes), the most likely candidate for destructive and transmissible new infectious diseases is the highly mutable influenza A virus; for that reason, a deeper examination of the flu virus follows.
Importance of Influenzas
Flu is a significant global infectious disease dangerous risk for several reasons. First, variations of the virus are highly likely to be transmissible by aerosolized (through the air) means and inhaled, or by contaminated surfaces. The virus also has the capability to spread easily among either human and/or animal populations. Thus, most strains of flu that infect humans do not require intimate contact to be passed on, since they can be passed on to secondary victims by either inhalation of airborne particles, or self-infection from contact with contaminated surfaces on which the relatively sturdy virus has been deposited.
Secondly, influenza is both highly “mutable” (prone to high levels of natural mutation) and able to recombine cross-species with other hosts, especially pigs and birds flu viruses, enabling human infection with dangerous novel strains of the virus. This has enabled historic changes that can make what might originally be solely bird or pig flu able to infect humans.
A closer look at the influenza virus helps us to understand why some epidemics are milder while others are more deadly. While there are three types of influenza viruses, it is Type A that kills the greatest number of people each year and is the only type that causes pandemics. Type A influenza originates in wild aquatic birds, which is where the term bird flu came from. When this virus is transmitted from wild birds to domestic fowl, it can undergo changes as it replicates in a new species. Moreover, the virus can also be transmitted to pigs from the chickens in a barnyard type setting and it mutates further; hence, the term swine flu is derived.
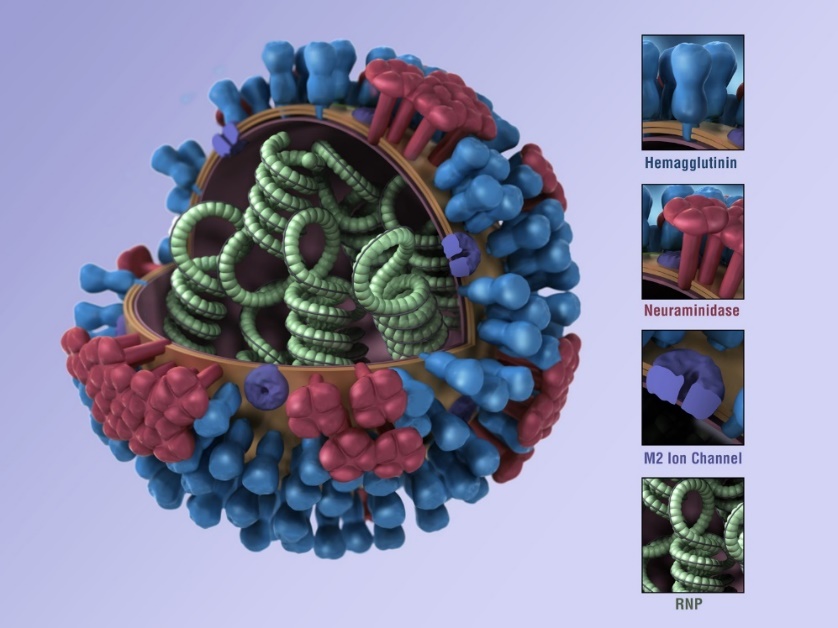
The genetic makeup of an influenza virus is a loose combination of RNA and protective proteins, which tend to rearrange when it infects a new species. This process can develop a new viral strain with the possibility of transmission to humans. Human herd immunity to a new strain is unlikely and that is when an epidemic can ignite. Another helpful piece of information is the naming of the virus. Flu viruses have two kinds of proteins on their outer surface, one protein's name begins with an H (hemaglutinin) and the other with an N (neuraminidase), which is illustrated in Figure 2. The name simply becomes a count of these outer proteins as identifiers, for example: H1N1, H5N1, or H3N2.
Third, hundreds of actual influenza strains exist along with potential genetic variations; some of which are primarily or even solely dangerous for one species. Still, many strains can infect and cause disease among more than one species. All influenza strains have the potential of sufficient genetic change to be more easily passed from or cause severe disease in one species to or another. For example, in spring of 2015 there was a deadly flu epidemic among commercial bird populations in several U.S. states. At this time, the particular strain of influenza was not a threat to humans, but it caused large-scale fowl deaths and led to further precautionary euthanasia of chickens, turkeys, and other commercial birds – causing a reported $1.2 billion avian flu economic damage in Iowa alone, including loss of over 8000 jobs (Wappes 2015).
Flu pandemics generally emerge when a novel strain emerges from one (or more) of the animal species most associated with interspecies exchange of the virus, mostly from pigs and birds – especially domestic chickens and ducks, though wild birds can also transfer these virus strains to the former, and thus help spread such strains around the world (CDC 2014). Figure 3 below illustrates the mutation pathway to cause human disease. This emergence of a new strain is important because the human population will have little or no preexisting immunity to it, and these strains can emerge through significant genetic shift – often through recombination between different strains (human and animal), or from natural mutations (Suarez et al., 2004).
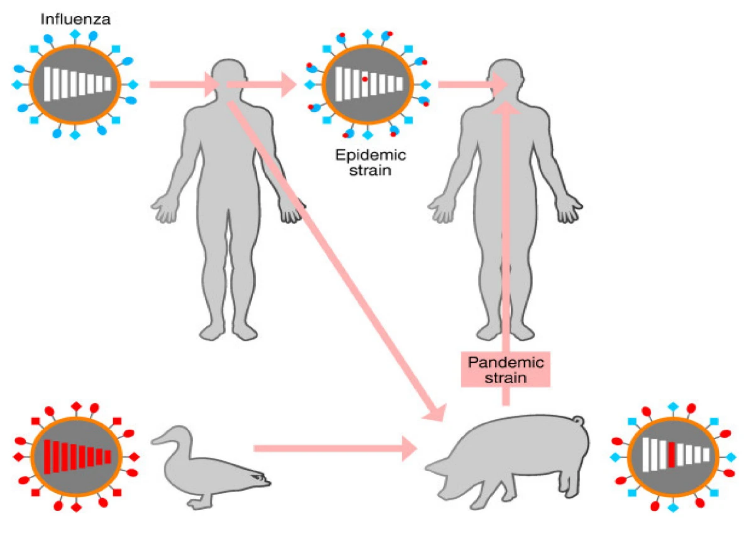
Influenza pandemics usually occur when a new strain of the influenza A virus emerges via antigenic shift and is transmitted to humans from another animal species. Species that are thought to be important in the emergence of new human strains are pigs, chickens and ducks. Unlike seasonally circulating flu strains that often share much of the same genetic profile, these novel strains are mostly unaffected by any immunity people may have to older strains of human influenza and can therefore cause more severe disease. Virulence, however, does not automatically mean such a strain will spread easily, transmissible, or infect very large numbers of people (WHO 2015).
Influenza remains among the most likely threats to humanity at the pandemic level. Considerable progress on universal vaccines for influenza has been made, but this promising research is still far from implementation (Nabel and Fauci 2010, Brown 2015). Therefore, public health measures must continue to focus on surveillance, containment measures, community participation, and learning along the way to improve these processes.
Global Surveillance and Response Systems
“Understanding the reasons why people die can help comprehend the ways people live, to improve services, and reduce preventable deaths” is a slogan coined by the World Health Organization (WHO) in reference to collecting data on morbidity and mortality. This data is the nuts and bolts of epidemiological function for learning more about disease patterns and the populations they affect. The ongoing analysis of the data informs both containment responses and development of policies for building more resilient communities.
Coordinated disease detection with appropriate containment measures is one of the best means to prevent the spread of ID. The broader Public Health role in infectious disease control consists of the following actions:
- Surveillance and tracking of infectious diseases.
- Identify pathogen agents.
- Provide prophylaxis and response as indicated.
- Implement social distancing, isolation and quarantine as the incident dictates.
- Coordinate the information and guidelines for medical providers and the public. (Feldmann-Jensen, 2014)
In the realm of communicable disease control, hyper-vigilance is necessary with data as the currency.
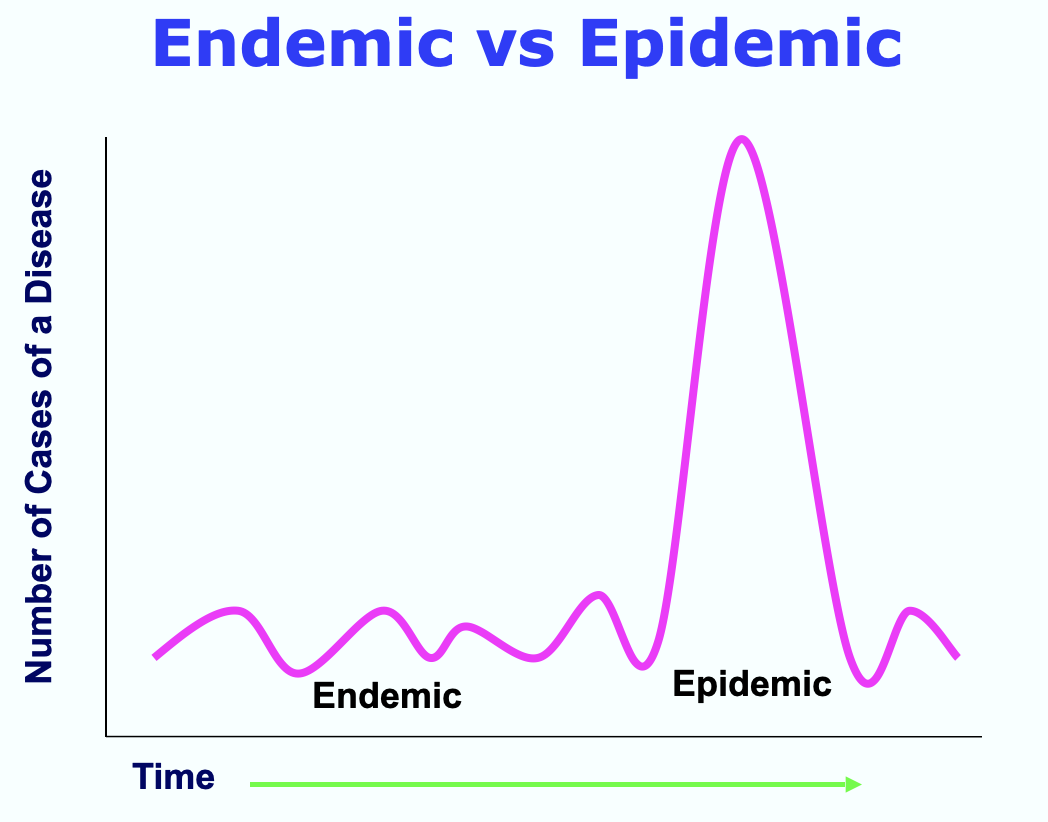
Clarity on ID patterns is critical to identify emerging syndromes early. It may be that a specific ID is persistently present in a particular geographic area, otherwise known as endemic. However, if the numbers of those affected rise beyond expected parameters, an epidemic is underway. Figure 4 below illustrates this difference. When an epidemic spreads globally to more than one continent, the outbreak becomes a pandemic. Significantly, as in the case of a well-controlled or eradicated ID (such as Smallpox), a single case would be considered an epidemic. These differences are important because they signal activation of containment responses and resource allocation requirements. Additionally, close data surveillance is vital in noting unusual patterns of ID emergence that can indicate an unfolding bioterrorism event.
Most local public health systems around the world are at a regional or municipal level, which is then coordinated at a national level, and sometimes a state or provincial level between. The local level is key in surveillance for unusual cases or rising cases of infection, as well as in beginning any containment and intervention measures. Optimally, the local data is reported to national levels and onward to the WHO, but this can be a slower flow. Thus, a global electronic epidemiological information sharing platform has also emerged to ensure rapid real time sharing of relevant cases and outbreaks.
The local systems are key because every event is local first. Engaging participation of local communities and multiple disciplines is vital to make inroads in the improvement of risk reduction in this context. Civil society still can create pressure from the bottom up, to make protecting environments and promoting health a topic of concern (Chan, 2010). This local action requires familiarity with historic infectious disease outbreaks and pandemics to recognize basic patterns.
Important Historic Outbreaks and Pandemics
Historic infectious disease disasters were both frequent and the leading cause of death before modern epidemiology and medicine. Among the greatest in history were the 18th and 19th century cholera and yellow fever pandemics, and the most infamous of all, the bubonic plague that depopulated almost half of Europe and the world in the 14th century. Three significant historic pandemics are discussed to provide context of the societal disruption they brought.
Plague: Black Death
Perhaps the most notorious of deadly and terrifying pandemic diseases in history is plague. The pathogenic Yersinia pestis bacteria is the causative agent generally associated with infected rodents (including squirrels) and transmitted by their fleas to humans. While normally treatable with antibiotics if discovered in time, plague can be fatal in 30-60 percent of untreated cases. Three different kinds of plague are known to exist in humans: bubonic, septicemic, and pneumonic. Bubonic plague is the most common infection and is usually transmitted by infected fleas, characterized by swollen lymph nodes, or "buboes.” The least common, septicemic plague is a bloodstream infection with early flu-like symptoms, including fever, chills and abdominal pain. The deadliest version, pneumonic plague, is a respiratory infection that’s the only one transmissible from person-to-person (mostly requiring relatively intimate contact), and the main cause of fatalities in the Black Death (CDC 2015c).
The mid-14th century “Black Death” was among the worst pandemics in history, emerging in Asia and killing one third to more than half of Europe’s population (Ziegler 2013). Subsequent epidemics periodically emerged through the centuries.
So infamous is plague that it has become a generic term for any devastating infectious outbreak. Human cases of plague are generally rare in developed countries, primarily because of modern public health, hygiene, and medical capacity – but especially garbage collection, rodent and insect control, careful reporting and monitoring, and other non-medical prevention measures (Kugeler 2015). Plague can be transmitted by infected domestic dogs (very rarely) and cats, the latter often by eating infected small wild animals (CDC 2015c).
Among the most sobering 20th century outbreaks likely tied to bubonic and pneumonic plague began in 1994 in Surat, in the Indian state of Gujrat, and spread to five other Indian states, killing at least 50 people. Despite some uncertainty about what the origins of the deadly epidemic was (Hazarika 1995), the Surat plague was notable because of its size and the panic that arose. Initial rumors of mass water poisoning and other speculative reports, followed by media announcements of plague and an unsuccessful effort to quarantine the city’s residence, led to an estimated 300,000 people – as much as 25 percent of the city -- to flee the city to neighboring regions. This panic led to refugees taking plague and/or plague vectors with them to neighboring areas, and to lurid international headlines and rumors about a looming “black death” emerging from India. Flights were canceled and Indian aircraft fumigated and quarantined. But the wide distribution of antibiotics and non-medical public health measures helped suppress the outbreak before it gained a foothold in major Indian cities, and in the end over 90 percent of the 53 reported fatalities occurred early in the outbreak, in Surat (Dutt et al. 2006).
In many developing nations with poor public health/medical and other service infrastructures, plague continues to periodically cause major outbreaks. Among recent epidemics, one 2014-15 outbreak in Madagascar killed more than 70 people.
For wealthy countries, Plague is potentially a future scourge, primarily because of the threat as a biological terrorism agent. Plague bacteria is one of six pathogenic organisms that are on the CDC’s Category A “Select Agents” list of the most dangerous potential bioweapons – in part because of its ease of acquisition, production, dispersal, and historic reputation for terror and death (Riedel 2005, Inglesby 2000). While weaponization of a pathogen can be ominous, global trading networks with the capacity to carry ID widely expanding its territory can be even more testing.
Globalization Comes Home to Roost: Cholera and Yellow Fever
The mosquito borne disease, Yellow Fever, was ubiquitous in tropical countries but had also periodically devastated temperate regions in Europe and North America during the 1700s and 1800s. A notable globalization influence, the disease was imported from ships arriving from the Caribbean and Africa, among other regions. Among the notable events was the 1793 Philadelphia yellow fever epidemic, which led to the deaths of more than 10 percent of the then-new United States capital city’s population and sparked the early American public health “sanitation movement” (Foster, Jenkins, and Toogood 1998).
Cholera was the first major pandemic disease for which the industrial revolution and global trade bears a major responsibility. The pathogen was spread from port-to-port by commercial shipping, and high death tolls brought misery, death, and major economic losses from disrupted commerce. Cholera is an acute water-born intestinal disease that is caused by the Vibrio cholerae bacteria. The pathogen has been one of modern history’s greatest scourges. In severe cases, it can kill in a matter of hours. Cholera has killed tens of millions since the early 19th century and persists today in periodic outbreaks.
Cholera helped motivate the world’s great trading powers to cooperate on a global scale in trying to minimize outbreaks and pandemics from those three diseases (including plague and yellow fever) in particular – leading to the first of a series of International Sanitary Conferences (ISC) in 1851 (Huber 2006). The ISC created rules and guidelines for quarantine and monitoring of global trade, which were hashed out. This cooperation and rulemaking, which began with cholera, eventually led to the current global public health system, coordinated by the World Health Organization (WHO) (Godlee 1994).
Cholera provided the impetus for over 50 years of International Sanitary Conferences in the latter half of the 1800s, in which rules and guidelines for quarantine and monitoring of global trade were hashed out. This cooperation and rulemaking, which began with cholera eventually led to the current global public health system, coordinated by the World Health Organization (Godlee 1994). This system becomes profoundly tested in the event of a pandemic of influenza.
The Elephant in the Room: Influenza
Influenza, while often not taken seriously, can become a devastating and crippling force to society when a pandemic of a virulent strain takes hold. The influenza virus is among the greatest known contemporary threats for a global pandemic infectious disease. Most people are accustomed to normal, seasonal flu, which while in an average year kills tens of thousands. But those deaths are usually among those who are very old, very young, or with preexisting medical conditions.
The world has experienced four flu pandemics in the last 100 years, including the 1918, 1957, 1968, and 2009 outbreaks. The deadliest influenza pandemic occurred from 1918-1919, infected as much as half of the human population (Patterson 1991), and killed anywhere from 50 to 100 million people around the world (as with many major deadly public health disasters, record keeping is often unreliable) – possibly as many total deaths, if not nearly proportionate to the population, as the 14th century Black Death plague. Pandemic flu is rarely as deadly as the 1918 Influenza but has the potential to spread widely (Knobler et al. 2005, Barry 2004). Significantly, “the 1918-1919 influenza pandemic was a defining event in the history of public health. The legacy of the 1918-1919 pandemic lives on in many ways, including the fact that the descendants of the 1918 H1N1virus have continued to circulate for nine decades” (Anthony Fauci, quoted in Morens, 2009).
Novel strains of influenza have been among the most common and notorious sources of periodic pandemic infectious disease outbreaks throughout history, and overall flu remains an ongoing global threat to both human public health and commerce (Taubenberger and Morens 2006). The goal is to anticipate and be prepared to respond accordingly when the new strain causes an outbreak.
In summary, the literature provides a contextual overview of public health ID concepts that are critical for the emergency manager to know. These concepts include terminology and the causality model- the epidemiological triangle. The model depicts how the interactions between the properties of the infectious agent, environmental factors, and characteristics of the host can determine outcomes. The multiplicity of human factors was explored in greater detail, as systems theory shows that disaster encompasses a significant human component. Established global systems provide a basic background on what is already in place for surveillance and response. Important historical pandemics were discussed giving deeper context for moving forward to the more current case studies.
Methodology
This chapter presents a case study format analysis of important outbreaks or incidents in the 21st century. The overall objective was to tease out the themes and lessons learned from the preparedness, response, and mitigation efforts in each case. As stated, the primary consideration for the respective case studies is that they occurred between the years 2000 and 2024. Secondly, the cases selected reflect current international capabilities and resources. Notably, not all outbreaks are equal because of complex interactions occurring between the emerging infectious agent, the physical environment, the built environment, human systems, as well as the biological factors of both humans and microbes. These interactions are shown below in Figure 5The convergence model, which depicts how interaction between humans, microbes, and the external factors (physical environment, ecology, socio-political, economic, genetic and biological) converge in a way that an infectious disease emerges. Accordingly, these key factors of outbreak roots inform the categories discussed below to further derive case selection criteria.
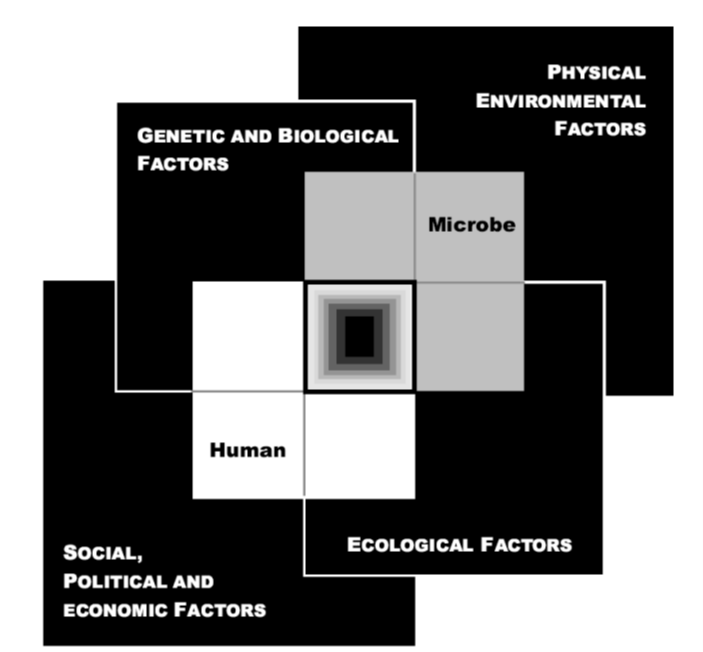
Key Roots of Outbreaks
Variability exists among outbreaks, influenced by the dynamic interdependencies and interactions between transmissibility, virulence, treatment options and agent source of origin. The interplay yields important differences, which is supported throughout the literature. These differences inform the categorization of outbreak distinction, as well as criteria for case study selection. Notably, the categories of significance are the more important aspect, with the individual cases discussed demonstrating the reality of societal disruption that specific variable can bring. The following ID categories of significance are among those highlighted in the literature:
-
- New emergence warns of future pandemic potential, while hardy systems contain it (Heyman, 2003; Morens, et al., 2004; Osterholm, 2007; Price-Smith, 2009).
- Eradicated infectious disease returns to an area due to globalization influence (Henderson, D.A., 2009; Price-Smith, A.T., 2009; Gates, 2022).
- The first pandemic of the 21st century (WHO 2005; Osterholm, 2007; Bell et al., 2009; Fisher, 2009; IOM, 2009).
- Vulnerable systems give opening to a regional outbreak and endemnicity (Farmer, 2004; Garrett, 2005; Gates, 2022).
- Rising threat as a bioterrorism agent with biotechnical and AI risk profile (Heyman, 2003; Guilleman, 2005; Lackoff & Collier, 2008).
- Agent is resistant to drug treatment (Heyman, 2004; Morens, et al., 2004).
- Recurring childhood agents that are vaccine preventable (Kluger, J., 2015, CDC, 2024).
- Population and urban encroachment into biodiverse zones that increase zoonotic transmission (Heyman, 2003; Morens, et al., 2004; Parums, 2023).
- Re-emerging vector-borne transmission with underlying globalization and climate change influence; and may have adapted to multiple species (Heyman, 2003; Morens, et al., 2004; Parums, 2023).
- An influential pandemic forshadowing likely future pandemics (Osterholm & Olshaker, 2020; Patrick, 2020).
Subsequently, this criterion was applied in the selection of cases presented and analyzed in case study format. Each case was relevant to a particular defining attribute of ID significance. The selected cases include: 2002 SARS, 2010 Cholera, 2012 MERS, 2001 Anthrax, 2009 H1N1 Influenza, 2013 Ebola, 2015 Zika, extremely drug resistant (XDR)Tuberculosis, Polio, and 2019-2023 COVID19. In the table below, the cases are correlated to the category of ID significance.
|
Category of Significance |
Representative Outbreak Case |
|---|---|
|
1. 1st Virulent Coronavirus Outbreak: Warning of future pandemic potential -Hardy Systems (barely) contained it
|
2002-3 SARS CoV1&2 |
|
2. 1st 21st Century example of an eradicated ID returning due to globalization influence
|
2010 Cholera outbreak, Haiti |
|
3. 1st Pandemic of the 21st Century
|
2009 Influenza H1N1 |
|
4. 2nd Virulent Coronavirus Emergence - Vulnerable Systems give opening to regional outbreak & endemnicity |
2012 MERS CoV |
|
5. Rising threat as bioterrorism agent – biotechnical and AI risk profile
|
2001 Anthrax |
|
6. Agent is resistant to drug treatment
|
MDR & XDR TB |
|
7. Re-occurring childhood agents that are vaccine preventable |
Polio |
|
8. Population and urban encroachment into biodiverse zones –zoonotic transmission
|
Ebola 2013 |
|
9. Re-emerging pandemic- like vector-borne example of transmission with underlying globalization and climate change influence; may have adapted to multiple species |
Dengue (most reported in >100 countries w/ 390 million annual cases) |
|
10. 3rd Virulent Coronavirus Emergence-Catastrophic Pandemic
|
2019-2023 COVID-19
|
The cases are presented individually with a description of the outbreak, the demographic effects, social behavioral health effects, economic impacts, and the disease management dynamics for containment within, across, and between borders. The case discussion subheadings for teasing out the themes in which to discuss the cases are validated influences derived from the literature review (Price-Smith, A.T., 2009; Lackoff & Collier, 2008; Lancet, 2017). The subcategories operational definitions for discussion are:
- Demographic effects -The human health outcomes resulting from the ID outbreak. These effects fall along a continuum from full recovery to long term morbidity to death.
- Social behavioral health effects – “The psychological repercussions of an outbreak, notably fear and anxiety, that impede decision making at the individual and collective level. Also includes the construction of the ‘other’, resulting in stigmatization, persecution of minorities, or violence” (Price-Smith, 2007, p. 20).
- Economic impacts - Direct and indirect monetary costs on families, businesses, and states, including reduction of foreign investments.
- Management dynamics for ID containment - Capacity for delivery of essential services. ID disruptions can shift power from people to the state, competition for scarce resources, destabilization yielding severe or coercive practices against the population (Price-Smith, 2007).
Each case discussed revealed key themes from the outbreak containment effort, which are noted at the end of the case discussion.
The second phase of analysis then explores the broader picture surrounding the outbreak cases. To do so, a qualitative analysis was conducted with the emergent themes across the categories of ID significance. The analysis of the themes derives comprehensive findings to further inform adaptive strategies shaping preparedness, response, and mitigation for future infectious disease outbreaks.
Case Study Results
Learning about the array of the 21st Century outbreaks frames the overall global bio risk situation. Further, a better understanding of biosecurity threats is informed by both technical elements and the underlying factors (Lackoff & Collier, 2008), as described in the methodology. Like other hazard events, the context of how a population’s social, economic, physical, and political environments interact with each other influences and even exacerbates an ID outbreak. Each of the case studies that follow represents one of the ten significant categories of infectious disease risk and are not in chronological order. The cases are discussed in terms of human effects as well as the management dynamics with that categorical factor. Emergent themes evidenced in each case are highlighted and then brought together to begin to frame the contemporary infectious disease risk situation.
Case 1: 2003 SARS CoV 1: A Warning of Future Pandemic Potential
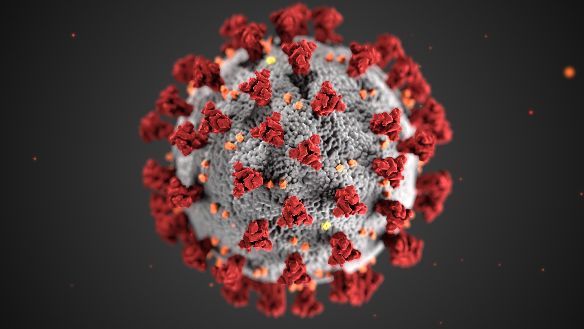
A poignant example of a warning of a future pandemic potential is the 2002-2004 outbreak of severe acute respiratory syndrome (SARS). The outbreak is among the most recent newly emerging viruses and was the first dangerous variety of the coronavirus (CoV) family to appear globally. The generic corona virus is illustrated below in Figure 6 and is relevant for the MERS and COVID-19 (discussed in cases below) virus structure as well.
This illustration was created at the Centers for Disease Control and Prevention (CDC). Note the spikes that adorn the outer surface of the virus, which impart the look of a corona surrounding the virion, when viewed electron microscopically (CDC-PHIL, 2024).
Outbreak Description
SARS-1 began with animal-to-human transmission and illustrates the problems with inadequate monitoring and communication during outbreaks. The novel SARS-CoV1 virus emerged for the first time ever in late 2002 from a bat population in southern China; the human interface began with a local open market in Guangdong Province selling wild animal meat in unsanitary conditions, including bats (the original virus reservoir), and secondary viral hosts wild civet cats, badger dogs, and pangolins for human consumption (Brüssow 2023).
The index case (refer to definitions) occurred in the Foshan, China, where it presented as a mysterious and deadly unknown respiratory virus (IOM, 2004). Institutes of Medicine (IOM) (2004) findings reveal that the provincial and national ministries of health investigated the outbreaks and found an atypical pneumonia, and they recommended measures to hospitals in the region for prevention and treatment of the infection, as well as a case reporting system be established. Unfortunately, this timing coincided with Chinese New Year, taking attention away as disease increasingly spread with holiday travel (IOM, 2004).
Transmission outside of China began a few weeks later in Hong Kong, where 12 people in a hotel contracted the virus from an infected Chinese visitor, whereupon some of them carried it with them to Singapore, Vietnam, Canada, and the USA (IOM, 2004, McGill 2015). In a matter of three days, SARS-CoV1 broke out in these countries and eventually on several continents. Modern technologies facilitated the rapid spread via airplane, building ventilation systems, and hospital clinical environments. Most worldwide cases originated with this Hong Kong superspreader event (IOM, 2004).
Demographic Effects
During its height, SARS had raised fear of a global pandemic, and while a pandemic never occurred, it did cause significant epidemics in Asia (particularly China, where it started) and Canada, and lesser outbreaks in Australia, Europe, Africa, and North and South America. The cities of Hong Kong, Singapore, and Toronto, Canada were among the most seriously affected.
Globally, SARS caused around 800 deaths among the 8,096 confirmed infected, making the case fatality rate (death rate of those infected) over10 percent before containment (IOM, 2024). Overall, the outbreak was significant due to the alarmingly high case fatality rate, identification of superspreaders, and the speed of global spread.
Psychological Effects
The uncertainty arising from a novel pathogen and the severity of illness and death produced sensational global media attention, which generated fear and anxiety throughout the world. Despite the relatively low number of deaths and relatively rapid containment, the outbreaks had a powerful and negative psychological impact in both infected and unaffected areas (IOM, 2004). Further, the initial lack of knowledge about the novel virus resulted in diagnostic errors and delays, putting healthcare and public health workers at risk, and adding additional stress, anxiety and stigmatization were common (Mauder, 2003 in Douglas et al., 2009). A study in Canada showed that in the outbreak’s immediate aftermath, there was PTSD in 28.9% and depression in 31.2% of the quarantined people (Douglas et al., 2009).
Economic Impacts
Ultimately, as with most such epidemics, fear and anxiety led to greater economic damage than direct morbidity and mortality. As a foreshadowing of Covid-19 disease responses years later, the SARS-CoV-1outbreak disrupted international travel and national economies and caused a crisis in global diplomacy and public health infrastructure (Knobler et al 2004). Even though it was mostly contained within several months, the behavioral effects (lockdowns, travel $40 billion dollars in global GDP loss (World Bank, 2014). Overall, the economic damage was viewed as moderate. Conversely, “the unexpected and unfamiliar disruption presented a socio-economic crisis for decision makers” (Price-Smith, 2009, p. 157).
International Infectious Disease Management Dynamics
The SARS-1 epidemic provides a glimpse into the significance of an EID as an “agent of destabilization internationally” (Price-Smith, 2009, p.140). One reason for the global public health diplomacy crisis was that in the initial months of the outbreak, the Chinese Democratic Republic’s government denied or minimized the growing problem, out of apparent concern that travel, and commerce would be disrupted. Beijing’s extensive secrecy about SARS dismayed the world, particularly global public health officials at the WHO. In response to Chinese obstruction of international infectious disease investigators, the WHO declared an unprecedented travel advisory and alerts for China. Notably, this was the first time WHO did so without the cooperation or authorization of the member state (Fidler, 2004), which led to subsequent revisions in the International Health Regulation, allowing WHO to do so formally (Fidler 2004).
The SARS outbreak exposed weaknesses in China’s public health preparedness, which included inadequate funding, no surveillance system, and shortages in prepared medical facilities and staff (IOM, 2004). The China problem was rooted in organizational obstacles, such as information flow and lack of coordination among siloed government departments and hierarchy (IOM, 2004). Notably, system failings, such as these, were evidenced in other countries in the SARS response. On the bright side, the outbreak did prompt the Chinese to establish a case reporting structure, strengthen its health response system, and provide funding for prevention and control of ID (IOM, 2004).
As with many such infectious diseases, the critical role of both early diagnosis and proper isolation and infection control measures is underscored (Li et al., 2004). As the international public health communities began to get news of the outbreak, WHO initiated and coordinated much of the response. On the other hand, multinational, collaborative, and coordinated surveillance, research, and containment measures greatly limited the spread (IOM, 2004). Partner organizations comprising 115 national health services, academic institutions, technical institutions worked together with the Global Outbreak Alert and Response Network (GOARN) (IOM, 2004). Importantly, a virtual network was created.
The IOM panel reviewing the SARS epidemics (2004) highlighted features of the response showing fundamental improvements in how the world responded to the outbreak, which was successfully contained almost a year after it first appeared. The SARS epidemics were also important because they made visible significant weaknesses in global/local preparedness for surprise outbreaks (Price-Smith, 2009, p. 140). Additionally, the complex interdependencies among countries were also evident in the case that can be important in preventing bioterror attacks (Price-Smith, 2009, p. 140). Clearly, the need for continued global investments to keep a response system prepared for the next emerging ID was underscored (IOM, 2004). Ultimately, the most important outcome of the SARS-COV-1 outbreak was the creation of related International Health Regulations and the update of the reportable disease list (Price-Smith, 2009, p. 156).
Case 1 Emergent Themes
- Revealed weaknesses in global and local preparedness for surprise outbreaks.
- Illustrated how an EID can rapidly destabilize society.
- Illuminated numerous interdependencies.
- Highlighted the need for continued investments in a response system.
- Functioned as a catalyst for change; e.g., Creation of International Health Regulations
Case 2: 2010 Cholera Outbreak, Haiti – Globalization Brings Back an ID Once Eliminated
Cholera is an ancient disease, controlled but not conquered in many parts of the world. The global economy and transportation play a key role in conveying the infection. Typically, Cholera is a “disease of impoverishment, displacement, and unrest” (Ryan, 2011, p. 2175). Vibrio Cholerae is a bacterium transmitted through fecal contamination of water or food. The waterborne bacterium causes severe watery diarrhea, leading to severe dehydration, shock, and death (MMWR, 2010). Yearly, around 100, 000 people die and 2-5 million people are affected by the infection (Ryan, 2011).
The devastating waterborne or foodborne disease is endemic to more than 50 countries; yet it can be avoided through effective sanitation and water treatment (Ryan, 2011). Vaccines are available but only provide short-term protection, and the microbe is everchanging and showing resistance to some anti-microbial drugs (Ryan, 2011).
Outbreak Description
The 2010 Cholera outbreak in Haiti was an example of globalization effects on populations. In 2010, Haiti experienced a devastating 2010 magnitude 7.0 earthquake. Notably, even before the ruinous earthquake, much of Haiti’s population lived in extreme poverty. Further, Haiti had pre-existing infrastructural deficiencies with significant water and sanitation problems (WHO, 2010). In addition, cholera had been eradicated from Haiti for more than 50 years and the population no longer had herd immunity; the conditions set it up for a re-entry. Consequently, the 2010-present cholera epidemic began shortly after the earthquake.
Cholera continues to demonstrate the potential perils and opportunities of globalized trade and travel. Ironically, the Haitian epidemic was traced to United Nations Nepalese humanitarian aid troops, who were brought in for earthquake relief. One or more was carrying the bacteria when they arrived and started the epidemic because of their improperly placed and constructed outhouse next to a Haitian water source (Orata et al., 2014). Thus, the protracted epidemic in Haiti was also part of a bigger picture: the effect of globalization on the world’s most vulnerable.
Demographic Effects
The first month of the epidemic alone had a total of 4,722 cholera cases and 303 deaths reported (MMWR, 2010). Just two years after the outbreak had started, over 6% of the Haitian population had already contracted cholera. Overtime, more than 820,000 Haitians had contracted the illness with 9,792 deaths reported (Griffiths et al., 2021). No confirmed cholera cases had occurred in Haiti between February 2019 (Griffiths et al., 2021) and October 2022. Figure 7 below illustrates a Haiti Cholera treatment site.
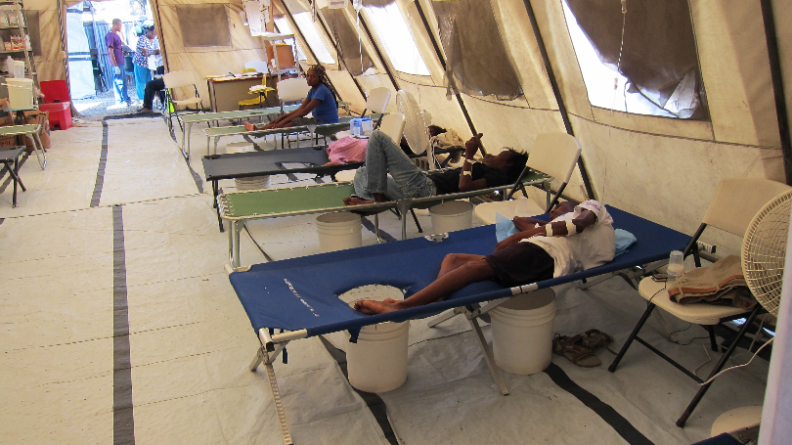
Psychological Effects
No mental health services existed in Haiti prior to the earthquake. Further, following disasters in the low resource area have had challenges coordinating services comprising mental health and psychosocial support (Raviola, et al., 2012). In 2010, a newly established mental health and psychosocial team was formed with Partners in Health to help with the widespread grief. The cholera outbreak increased the demand for such teams. Initial funding to generate a wider system came from several foundations.
The epidemic brough suffering, death, grief, and even greater fear following the losses of the earthquake and its aftershocks. The poor water and sanitation along with minimal health services compounded the situation. Those ill with cholera became stigmatized and abandoned.
Economic Impacts
Haiti remains one of the most fragile and vulnerable countries in the world. The country has faced a series of human and infrastructure losses. As a result, both life expectancy and DALYs continue to decline. Looking to eradicate the cholera problem in Haiti, the Revised Haiti Humanitarian Plan of 2017-18 estimated cholera programming requirements for Haiti at US $21.7 million.
International Infectious Disease Management Dynamics
The case of the cholera epidemic in Haiti is integrally related to global cholera milestones (Ryan, 2011). As many people still lack access to safe drinking water and sanitation, economic investment and civil stability could do much to further the goal of defeating cholera both in Haiti and around the world.
Collaboration has been essential among international agencies and local non-governmental organizations (NGOs) to build local capacity, train domestic health care workers, and slowly build local resilience. During the epidemic peak, many cholera treatment sites were established. More currently, the focus is on “hygiene awareness and prevention strategies in localities with known high-risk factors, help concentrate limited resources, and improve efficiency in the fight against future cholera epidemics” (Griffiths et al., 2021). Given that the Cholera epidemic in Haiti has been ongoing for more than decade, ample opportunity has been given all stakeholders to learn, adapt, and respond to control and reduce cholera.
Case 2 Emergent Themes
- Reinforced hygiene awareness and prevention strategies in localities with known high-risk factors can help concentrate limited resources (Griffiths et al., 2021).
- Highlighted the essential need for investment in water and sanitation.
- Illuminated interdependencies; Cholera spreads through global interactions but affects those that benefit least from globalization (Ryan, 2011).
Case 3: 2009 H1N1 Influenza Pandemic - First Acute Pandemic of the 21st Century
The influenza virus is among the greatest known contemporary threats for a global pandemic infectious disease. Beyond the obvious health concerns, an influenza pandemic can also impact every level of society with implications for economy, infrastructure and the stability of society (Feldmann-Jensen & Kim, 2024). The first pandemic of the 21st century was both anticipated and a surprise.
Outbreak Description
The world was startled in the spring of 2009 by the emergence of a new influenza virus. For nearly a decade, a source of concern and heightened surveillance was focused on the influenza H5N1 virus, keeping attention on the birds of Asia. A new and unanticipated influenza outbreak began in Mexico, emerging through the pig population spreading to humans. The new H1N1 subtype (a version of the strain that caused the 1918-19 pandemic) proved to be quite contagious and rapidly spread from human to human.
One month later, numerous areas in Mexico were reporting influenza-like-illness (ILI), prompting the General Directorate of Epidemiology of Mexico to augment surveillance throughout Mexico and to convey the situation onward to the Pan American Health Organization (PAHO). Laboratory confirmed novel H1N1 was communicated to PAHO by the end of April (Feldmann-Jensen & Kim, 2024).
Mexico City had an Influenza Pandemic Response Plan in place for several years prior to the H1N1 emergence. Mexico City did utilize their existing stockpile of antiviral medications in attempts to contain transmission. Public health officials set up call centers and established public information campaigns. Hand and cough hygiene was encouraged, social distancing measures were implemented, surgical masks were distributed, public gatherings placed on hold, and soccer matches were televised only (IOM, 2009).
Public health capacity was the key challenge during the outbreak in Mexico. Healthcare was provided by 3 major healthcare systems in Mexico; therefore, compilation of epidemiologic information regarding hospitalizations was complex (Bell et al, 2009). The capacity for epidemiological surveillance and laboratory diagnostics was insufficient. Nevertheless, heroic efforts were made that saved lives (IOM, 2009).
The timing of the virus epidemic in Mexico was concurrent with the U.S.A. schools’ spring break, where Mexico is the most popular travel destination. In a few short days the influenza strain was seen in the U.S.A. New York City was the first place the novel virus was detected in the U.S.A., followed by Texas, California, and Canada. The spread of the virus ultimately found its way to 74 countries when the W.H.O. declared the outbreak a pandemic in June of 2009. Mexico was not the only place with capacity challenges. Many public health agencies around the world were affected by the simultaneous global economic depression; and were facing funding cuts and a range of human resource shortages.
As with anything unknown, much uncertainty existed in the early days of the outbreak. Questions abounded: ‘What characteristics did this new virus have?’; ‘How deadly is it?’; ‘Will a second or third wave of illness occur?’; and ‘When will a vaccine or other measures be available for mass dispensing?’. These questions take time to research, and time meant a chance for viral spread, people infected, sick, hospitalized, and lives lost.
The planning assumption had been an abundance of vaccine; in reality, vaccine production lagged, and demand increased. The spring emergence meant that the strain was not a part of the recent seasonal flu vaccine. Because vaccine production was not profitable in 2009, the processes had not been invested in for updated improvement and continued to function with old technology. The pandemic’s second wave was anticipated for the autumn of 2009; a key priority was having the vaccine in time to reduce the surge of the second wave. Creating a vaccine and then ensuring it was safe became a race against time.
Demographic Effects
The CDC (2024) estimates 151,700-575,400 people died from H1N1 worldwide during the first year. But as with other historic pandemic flu outbreaks, including 1918, it caused more severe disease in young adults, a population not badly affected by seasonal influenza because of their robust immune systems. Globally 80% of the H1N1 related deaths were estimated to have occurred in people younger than 65; mostly affecting children and younger adults.
A noteworthy factor that contributed to high-risk complications from an H1N1 infection, as it has for so many infectious diseases, was underlying health conditions, such as metabolic diseases (heart disease, diabetes, etc.), COPD lung disease, and cancers. These non-communicable illnesses significantly affected the mortality from H1N1.
The H1N1 pandemic underscored disparities worldwide in terms of understanding disease processes, capacities for response, resources available and the socioeconomic effects. These effects are seen in the inconsistent case fatality rates, for example 4% in Mexico and 0.1% in the U.S.A. The outbreak led to regional spikes in severe disease in some regions around the world. A need to provide equitable access to resources was evidenced for this and other emerging pandemics.
Psychological Effects
H1N1 responses revealed large regional differences in anxiety. As an example, Malaysians were generally more concerned and likely to reduce travel and buy masks and food (Goodwin, et al., 2009), while Europeans underestimated mortality and required more information to encourage vaccine uptake (Goodwin et al., 2009). Of additional significance, socially marginalized groups had a higher risk of infection, which calls into question equitable treatment and stigmatization (Goodwin, et al.2009).
Economic Impacts
The costs of a normal influenza season in the U.S.A. evidenced a mortality rate between 35,000-50,000, and more than 200,000 hospitalizations. This normal flu season costs approximately twelve billion dollars annually in direct medical costs and lost productivity (Garrett, 2005, p. 4). A moderate epidemic is estimated to increase these costs by twenty-five percent.
The real cost of the 2009-2010 H1N1 pandemic was 0.5% to 1.5% of GDP in affected countries. The World Bank estimated the global cost to have been between $45 billion and $55 billion. Losses reflect both the direct costs of morbidity and mortality as well as diminished transport, tourism, and influences on wider industries. Notably, this pandemic occurred concurrently with a global financial crisis; therefore, some of the effects are difficult to separate.
The global financial crisis is also attributed to reductions in the public health workforce. Public Health has long limped by on a patchwork of grants, which do not necessarily enable core public health functions. The inadequacy of sustainable funding undermines essential public health function and emergency response capacity.
International Infectious Disease Management Dynamics
The international cooperative effort was remarkable. Plans and policy were in place for an Influenza pandemic at all levels of governance. At the global level, the WHO had a Global Influenza Preparedness Plan to integrate and coordinate an international response. The plan established a Pandemic Phase Alert system. However, the Pandemic Severity Index had been designed for a more virulent virus, which caused some confusion for decision making at more local levels. Consequently, social distancing measures, school closures, handling of the worried well and use of stockpiled antivirals were inconsistent in implementation.
A global surveillance system was in place to monitor for outbreaks, yet minimal surge capacity existed. Surveillance was ongoing and elevated during H1N1. Still, the inadequacy of epidemiological data was noted in a review of the global surveillance efforts by the Institutes of Medicine (IOM), recommending the completeness and quality of data to support an evidence-based response. Surveillance also has an animal disease detection facet. While amplified surveillance among global the bird population was in place, careful monitoring of pigs was not.
Investment and policy were in place for the stockpile of antiviral medications to be used in the containment of an outbreak in the first affected areas. The goal being containment and elimination of the outbreak at its source. Countries were encouraged to stockpile antivirals prior to the pandemic, because medication manufacturing is lengthy, and the availability is limited in a crisis.
The response system demonstrated the capacity to produce a vaccine and conduct a mass vaccination campaign, which was critical in reducing the effects of the pandemic. Vaccine access globally had some difficulties, particularly for developing countries. The WHO engaged in negotiations to secure vaccines for these countries; this vaccine was distributed first to health workers in the least developed countries (IOM, 2009). WHO had an estimate that only 14% of the world was vaccinated one year after a severe pandemic began. This outcome reinforced the need for research investment for vaccines.
Case 3 Emerging Themes
- Verified that investments in pandemic planning and medication stockpiling pay off, with the caveat that plans must be adaptable to new information.
- Revealed that public health departments did not have enough resources to carry out plans.
- Exposed inadequacy of surveillance data.
- Revealed that the WHO pandemic alert phases caused confusion for decision makers.
- Illuminated complex interdependencies in international coordination.
- Demonstrated how even in a mild pandemic, health care systems were already overwhelmed.
- Highlighted the need for investment to modernize vaccine production.
- Proved how straightforward information to the public was essential for allaying fears and building trust.
Case 4: 2012 MERS CoV - Another Coronavirus interacting with Vulnerable Systems
Aside from SARS-2/Covid-19, among the relatively recent, newly emerging viruses have been two other novel, dangerous varieties of the coronavirus (CoV) family: SARS-1 and MERS – both of which also began with animal-to-human transmission, illustrate the problems with inadequate monitoring and communication during outbreaks, and pose continuing potential pandemic risk as such viruses mutate and evolve (Zumla et al., 2024).
Outbreak Description
The Middle Eastern Respiratory Syndrome (MERS-CoV) coronavirus, a cousin of SARS-CoV-1 and -2 (Covid-19), originated in Saudi Arabia in 2012 to great international consternation (Schnirring 2015). MERS was initially transmitted from camels and was limited to a few hundred cases in until May 2015, when a single case imported by a traveler returning from the Middle East started a cascading outbreak of human-to-human transmission in South Korea. The sick traveler became a “super-spreader,” going from one to another health-care facility and waiting for hours at each, and infecting dozens (Stein 2010).
Super spreaders are now common in outbreaks, including the SARS-CoV-2 (Covid-19) pandemic, where a small minority is implicated in most cases. They either transmit higher amounts of pathogen for genetic or other reasons (older, overweight, immune-compromised, etc.), or because of environmental conditions – what has been call the “80/20” rule (Lewis 2021). As with the first (2003) SARS-1, there was no evidence of widespread airborne (aerosol) transmission, and community-based human-to-human droplet transmission was the main culprit (Killerby et al. 2020). Globally, by mid-2015, MERS-CoV had infected more than 2,600 and killed over 950 around the world (over 36 percent case-fatality rate for confirmed cases) (WHO June 20, July 28, 2015).
Demographic Effects
The 2012 S. Korean outbreak had 86 confirmed cases and killed at least 36 (19% case-fatality rate). Migration and travel patterns of people, such as the single South Korean citizen superspreader, who brought MERS to his country in 2012, highlight the risk of globalized human movement in general, including combined effects of rapid, far-reaching airline travel; international shipping and cruise lines; and increasingly susceptible aging populations. The median age of South Korean MERS patients was 55 years (range, 16 to 86), and 55 percent had one or more coexisting medical conditions (Choi et al., 2016).
Psychological Effects
Even though comparatively small, the 2012 S. Korean outbreak had an outsized effect that led to weeks of panic and the isolation or quarantine of tens of thousands, before being controlled (Choi et al., 2016). Most residents wore masks in public, and large numbers of schools cancelled classes to protect their students (contrary to World Health Organization recommendations at the time) (Ha 2016).
The psychological impact of the first major coronavirus, the 2003 SARS-1 outbreak in Hong Kong, Singapore, and Toronto Canada in particular, was also substantial – and presaged what was to come for both the MERS outbreak and Covid-19 pandemic. Toronto’s SARS-1 epidemic went in two waves, and infected and in some instances killed healthcare workers and family members. But the time the second wave was over, hospital providers and staff were reported to be on the verge of walking off the job, and years later many were still suffering PTSD from the experience (Lancee et al., 2008).
Economic Impacts
In South Korea, estimated MERS-related tourism losses from 2015 on, with 2.1 million fewer noncitizen visitors was around US$2.6 billion – costing significant job impacts in the hospitality and travel sectors, and major temporary disruption to the emergency services sector (Joo et al., 2019). Added to this is under-resourced and/or poorly structured public health and medical systems that magnify the risk.
International Infectious Disease Management Dynamics
Another self-evident effect pre-Covid-19 was that despite historic examples of globalization-related mobility of pathogens, including 2003’s SARS-CoV-1, a country like South Korea was not prepared for a novel but already identified virus like MERS. Indeed, given its incentive structure (long waits, and patients regularly going from one emergency department to another hoping to get seen) the medical system’s unique structure made it ripe for nosocomial/medical transmissions. Given the disparate survival rates between the Middle Eastern cases and those in S. Korea, as is evidence in most infectious disease outbreaks, including pandemic diseases like Covid-19 and Ebola, access to modern critical care is essential for improved outcomes. In S. Korea, supportive care and antiviral therapy was administered to 75 percent of the confirmed cases, together likely lowered the 30-40 percent case-fatality rate MERS had had up to then (McGill 2015).
Case 4 Emergent Themes
- Evidenced a dangerous, potentially pandemic candidate virus, should it mutate to become more easily transmissible.
- Disrupted and necessitated complicated crisis management in the societies affected.
- Illustrated ongoing gaps in speed and effectiveness of both international and domestic public health response.
- Highlighted the importance of poorly managed super-spreaders.
- Revealed a limited medical surge capacity, including crowded hospitals with long waits and poor infection control enforcement.
- Damaged the economy, particularly because of reliance on tourism.
- Discovered unique outbreak amplifiers related to cultural habits.
Case 5: 2001 Anthrax and Rising Threat as Bioterrorism Agent
Among the most significant growing biological security adaptation dilemmas is from biowarfare agents. Not only must the world increasingly prepare for pandemic disease, but also for hazards from existing pathogens that are converted into weapons as well as the prospects of designer disease agents bioengineered using modern, easily available new genetic manipulation/alteration tools. The case of the 2001 anthrax attacks is just one example of a bioweapon agent that’s been used to kill and spread fear, but it also exemplifies the wicked, insoluble problems with even existing “naturally occurring” disease organisms being used for ill-purpose.
Outbreak Description
Anthrax is among the oldest and deadliest pathogens known to humankind. Although anthrax disease (from the bacillus anthracis bacteria) is not transmitted from person to person, epidemics, especially from consumption of diseased livestock, plagued societies back to pre-history and highly lethal inhalation anthrax is still among the most feared biological terrorism/warfare scenarios for weaponized (freeze-dried, precision ground, and treated with anti-static coating) existing disease agents (vs. potential bio-engineered new ones, discussed below). Anthrax is particularly problematic for response and planning as a bioweapon because it is easier to produce and handle (as a bacteria), and if inhaled in a powdered weaponized form can cause symptoms in less than 36 hours, at which point the prognosis is very poor even if treated with antibiotics (Patrick, 1996, 1999).
The 1975 Biological Weapons Convention (BWC) prohibits the research, production, and use of any bioweapon. Yet, several nations – especially the former Soviet Union – produced and stockpiled literally tons of that and other weapons-grade bio-agents for years after (Bower et al. 2022). Among the ways this was confirmed was after an accidental 1979 Soviet anthrax bioweapons facility release in which at least 77 in the city of Sverdlovsk became ill from inhalation anthrax, and 66 died, illustrating its deadly potential. Together with the testimony of former Soviet bioweapons officials just before and after the Cold War ended, who confirmed the vast extent of a program that had employed tens of thousands of people dozens of bioweapons facilities across the USSR. And tons of bioagent after the Soviet empire broke up around 1990 was never publicly accounted for (Meselson et al., 1994).
In the weeks after the U.S. September 11th, 2001, al Qaeda attacks, an event unfolded that few Americans remember now – though at the time it greatly amplified American fears of terrorism. Mailed envelopes containing small amounts of dried, treated, powdered anthrax were delivered to Congressional and press offices – infecting 22 people in total. Those exposed in this attack, including Postal Service workers infected at mail sorting centers, produced 11 inhalation cases with 5 deaths and 11 surviving cutaneous/skin anthrax cases. Thus, it was dubbed by some as the “5/11” attacks. Hundreds were evaluated to “rule out” anthrax, and as many as 30,000 advised to start precautionary antibiotics. 10,000 people in Congressional offices and those potentially exposed elsewhere were recommended to take at least 60 days of antibiotic therapy – since anthrax bacteria can “hide” in the body for up to two months, before emerging as a disease. But the majority did not finish the full course due to burdensome gastric side effects of the drugs. Meanwhile, hundreds of thousands more were affected by closures – including very expensive decontamination/cleanup of facilities, mail delivery disruptions, etc. The reputation of both the CDC and FBI were damaged by the investigation and contradictory messaging (Gostin and Nuzzo 2021; Cole 2008).
Demographic Effects
As with so many infectious disease threats, those who are most susceptible do biological attacks include the very young and very old, those with pre-existing conditions/comorbidities (including malnutrition), and the poor who have less access to medical care or monitoring.
Psychological Effects
The terror that the 2001 anthrax attacks generated spread across the globe, not just in the United States. One of the infamous patterns was thousands of “white powder incidents,” where average citizens mistook any powdered substance (including talcum and baby powder, and corn flower – then used to keep magazines dry in clear plastic mailers) for anthrax, or those seeking to harass or terrorize perpetrated copy-cat white powder mailings claiming it to be anthrax. Many of these incidents, especially initially in disparate U.S. and international communities, and then especially when mailed to sensitive and/ or government facilities, also triggered expensive and elaborate shutdowns and decontamination efforts.
Economic Impacts
The relatively small 2001 “5/11” attacks cost an estimated $320 million for decontamination alone (Schmitt & Zacchia, 2012). While biological weapons can potentially cause large-scale disease and death, they are often overlooked for their massive economic impact. In a larger-scale anthrax attack on a major city, for instance, it is certain that related decontamination efforts could easily cost $100s of billions (or more) because of the gap between “expert” public health/environmental mitigation standards and public aversion to any risk from such agents. People are notoriously inept at accurate risk perception of such hazards (Burns 2008), and even an infinitesimal remaining risk of exposure to post-decon trace anthrax or other persistent bioagent would dissuade at least a significant percentage of the public from returning to an area (especially those with children) – causing further, rippling, long-term economic damage (O’Sullivan & Ramsay, 2020).
Critics of extensive biodefense spending are concerned about the many $10s of billions that have been spent on preparedness and response programs for what some consider low probability bioterrorism events, to the detriment of higher probability naturally occurring pathogenic events (Shelton et al., 2012). But others “feel that these advances justify the substantial biodefence funding trend of the past two decades and set a precedent for future funding” (Long & Marzi, 2021). This view argues the broader legacy of biodefense spending has created – analogous to the 1960s U.S. space program, or other defense spending – dual use advancements in civilian public health biosecurity against naturally occurring pathogens like Covid-19, improved bio-surveillence, diagnostics, vaccine technology, and medical countermeasures (ibid).
International Infectious Disease Management Dynamics
In 2008, the U.S. Commission on Weapons of Mass Destruction reported that the most likely serious global or U.S. WMD threat would come from biological weapons – and suggested a 50 percent chance attack would occur by 2015 (WMD Commission 2008).[1] Even though such an attack has not (yet) happened, in recent years since the Amerithrax letters, a relative complacency has set in, despite hundreds of billions of dollars have been spent on international detection and countermeasures.
Exemplifying Western nations’ bioweapons risk management, U.S. biosecurity programs now include the Biowatch (urban bioterrorism detection), the U.S. Strategic Stockpile of drugs, vaccines, and EM response materiel; the BARDA drug countermeasure development program (mobilized during Covid-19); the Cities’ Readiness Initiative that prepares major U.S. urban areas for disasters, and other infrastructure. And as shown below in Figure 8, the United States CDC maintains a Category A (able to cause the biggest societal, medical, and EM challenges), B, and C list of potential bioweapon pathogens.
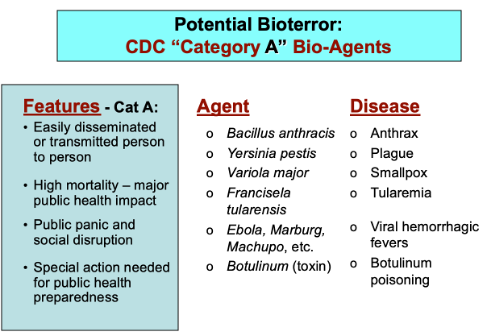
Part of the rising biological terrorism threat is exacerbated by the biotech revolution. “Dual use” biotechnologies such as genomics and related CRISPR gene editing, rapid PCR replication of genetic material, nanotechnology, and other genetic engineering is making it easier to manipulate or design pathogens, and while few aspiring terrorists would go to such lengths, there are enough well-funded and motivated ones to mandate a more robust international management of this risk (see e.g., Berger, 2021). Another related scientific and policy debate is how much of a risk potential “gain of function” research (testing what genetic changes might make a pathogen more dangerous) poses. Covid-19 highlighted this possibility of accidental release of an enhanced supervirus that might itself create the pandemic it was attempting to preempt (Berche, 2023) – and there have been numerous previous lab lapses (researcher infections or unsecured movement of pathogens) in biosecurity research labs. For example, in June 2014 as many as 75 CDC scientists were accidentally exposed to anthrax, and weeks later the U.S. Food and Drug Administration discovered 16 vials of smallpox virus (dating back to the 1950s) in a storage room on the National Institutes of Health campus, during a routine lab cleanup, that had been forgotten in storage (Stern & Schouten, 2016).
Coincidental with the gain-of-function debates, in October 2011 the Bipartisan WMD Terrorism Research Center released a report that gave a “report card” grade for various aspects of U.S. biosecurity preparation and capabilities, and based on broad, scaled scenario types, from smaller to larger. “Today we face the very real possibility that outbreaks of disease, naturally occurring or man-made, can change the very nature of America – our economy, our government, and our social structure” they began. Yet, with the exception of “communication,” each of the assessed categories was given D and F grades (Graham & Talent, 2010).
The above issues were already being robustly debated before Covid-19, as in 2006 and 2007 when two National Institutes of Health (NIH) National Science Advisory Board for Biosecurity (NSABB) reports highlighted dual-use and synthetic biology tech research risks, including de novo (from scratch) synthesis of select agents (NRC 2009). “Converging” biological- and nanotechnology (B-N) advances will be one of the great change variables in the twenty-first century – producing amazing scientific advances with a variety of beneficial applications, as well as substantial traditional and non-traditional security risks. This potential has been highlighted in numerous government and academic studies and reports over the last twenty years or more, in numerous government and academic studies (e.g., Tucker & Zilinskas, 2006; Relman, 2010; NRC, 2010).
Case 5 Emergent Themes
- Revealed that overall public risk perception is often overlooked in biological terrorism scenarios, especially at a complex systems level.
- Verified that a weaponized bioagent can become a far more rapidly critical and deadly outbreak than a naturally occurring outbreak of the same pathogen.
- Evidenced a need for a rapid response for mass casualty inhalation exposure to weaponized Category A bioagents.
- Signaled that scientific research and breakthroughs can pose a risk of accidental or intentional release of a laboratory enhanced virus.
- Incentivized a controversial limitation placed on publication of bio research that presents greater risks than benefits.
- Highlighted that bioweapons and biowarfare will become an increasing global security risk.
Case 6: Tuberculosis – Resistance to Multiple Drug Treatments
Tuberculosis (TB) is another ancient infectious disease that remains one of the leading infectious causes of death globally. Micobacterium tuberculosis has been called the most successful pathogen in human history, given its ability to hide dormant in infected people until their immune systems are weak and susceptible from age, poor nutrition, or other diseases. History shows us that TB thrives when people are crowded into small spaces and inadequate nutrition (Farmer, 2005, p. 120). Essentially, TB has been known as a disease of poverty, prisons, and war. Importantly, TB is both curable and preventable!
Tuberculosis is spread via droplets through the air from one person to another. Because TB transmission is airborne and the agent infectious, means that it is NOT exclusively a disease of the poor. Lungs are the primary place the infection sets in, but it can also affect other parts of the body. Figure 9 below illustrates how TB is seen in a lung x-ray.
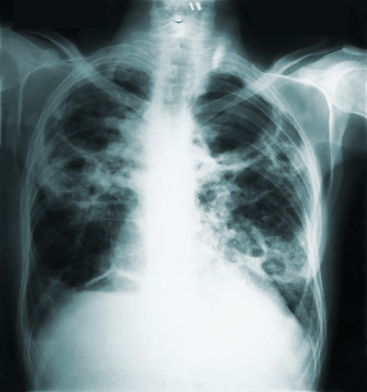
An estimated one third of the world’s population is infected with latent or dormant TB, and 10% of those will at some point experience an active case of TB. For that reason, TB often flourishes in tandem with HIV-AIDS, malaria, and other immune system-compromising infectious diseases.
Micobacterium tuberculosis, like other bacteria and viruses, is capable of mutating and adapting its environment. The rise of multi-drug resistant strains (MDR TB) and extremely drug resistant (XDR TB) strains present an ongoing public health crisis and are considered a health security threat.
Not long after TB treatment drugs were introduced in the 1940s, drug resistant TB was detected (Wingsrichanalai, et al., 2010). Drug resistance primarily arises from inadequate or inappropriate use of the anti-microbial drugs when treating TB. Misuse examples that contribute to drug resistance can include: 1) people not completing a full treatment course or do not take the TB drugs in an uninterrupted way, 2) provider prescribing the incorrect dose or length of time, 3) proper treatment drugs are not available, or 4) drugs are of a poor quality (CDC, 2024). These conditions are exacerbated in developing countries with under-resourced public health systems.
Outbreak Description
MDR-TB is defined by resistance to at least two TB treatment drugs, usually isoniazide and rifampin. Spread as readily as other versions, MDRTB is far more difficult and expensive to treat. MDR-TB has been a growing threat in much of the world, though primarily in low- and middle-income countries. Notably, half the new drug resistant TB cases occur in the BRICS nations, which include Brazil, Russia, India, China, South Africa, Egypt, Ethiopia, Iran, and the United Arab Emirates.
The case centers on the 30-year TB epidemic in the Russian Federation, the country with the largest burden of MDR TB in the world. Through most of the 20th century, the TB rates were falling in the former Soviet Union until the fall of the Berlin wall (Farmer, 2005). Cold-war era travel restrictions were eliminated, with business and tourist travel burgeoning. The global movement facilitated both TB and MDR TB to infect other parts of the world. The dismantling of both the Soviet-era health care and public health system and the social safety net in the workforce occurred in tandem. With the checks of the party system removed, the underlying corruption outpaced the democratization processes, opening the way to “violations of economic and social rights” (Farmer, 2005). This situation reverted to an age-old practice of arresting and imprisoning the impoverished; in fact, the rates of imprisonment doubled after the fall of the former Soviet Union. It was in these circumstances that gave TB disease a new foothold in the country. Shortfalls that directly related to TB were out of stock drugs, weakened civilian TB services, and failure to pay prison officials (Famer, 2005). TB rates tripled between 1990 and 1996. Alarmingly, Russia had 111,075 new TB cases occur in 1996 alone (Farmer, 2005). But the silent and very deadly problem was in its prisons where the TB rates were exponentially higher (Farmer, 2005). The inconsistent availability of TB drugs, services, and food within the prisons provided the conditions for drug resistant strains to evolve. Tuberculosis was once again the leading cause of death in young prisoners (Farmer, 2005). If by chance a prisoner survived to be released, they took the infection to others in the community they live in. The languishing situation continued to incubate and transmit MDR TB for many years.
A more current look at factors influencing MDR TB in Russia reveals that incarceration and treatment history remain the major factor influencing MDR TB prevalence (Bykov et al., 2022, p. 12). Bykov et al. (2022) explains a contradiction of Russian healthcare laws contribute to the prison conundrum. Of note, is a legal limitation for conducting epidemiological surveillance on infected patients, as well as former and current inmates, who all appear to be the main source of MDR TB (Bykov et al., 2022). Other highly important variables are despair driven IV drug use and HIV/AIDS coinfections as an epidemic amplifier. Nevertheless, a decrease in mortality is now evidenced, along with improved treatment and compliance among the incarcerated (Bykov et al., 2022).
Notably, “even more dangerous versions have emerged in all regions of the world. Extremely drug resistant (XDR TB), which is defined as resistant to at least three of the four of the mainline treatment drugs” (Parida et al., 2015, p.38) and resistant to at least one second-line drug. Because this type is resistant to the most effective drugs, the infection has a 50% survivability chance. Curiously, XDR TB strains have been found to be genetically diverse related to treatment context, which suggests that a single genotype is not being diffused (Hasan et al., 2010). The complexity presented has far greater treatment challenges. The most recent emergence is the incurable and totally drug-resistant (TDR TB) strains. Thus far almost all infections with this strain involves co-infection with HIV. TDR TB has a 98% mortality rate, usually within less than two weeks from diagnosis.
Demographic Effects
TB is present in all countries and all age groups. The global burden of TB disease reflects a disregard for human dignity of all people. Illness caused by TB in 2022 affected 10.6 million people. More staggering is the idea that only two-thirds of all cases are reported (Mello et al., 2018). Figure 10 below illustrates the global distribution of TB incidence. Deaths attributed to TB in 2022 were estimated at 1.3 million. TB was the second leading infectious disease killer globally, right behind COVID19 (WHO, 2023). Further, the COVID19 pandemic was a contributor to a new increase in TB rates globally (Starshinova et al., 2022).
Bringing the focus more specifically to the burden caused by resistant strains, MDR TB caused an estimated 160, 000 deaths in 2022. The estimated worldwide number of people who developed MDRTB remained even between 2020 and 2022, after a slow downward trend between 2015 and 2019 (WHO, 2023). The proportion of MDR TB cases to all TB cases was estimated at 4.0% in 2015 and fell to 3.3% in 2022. Figure 11 below reflects this ratio of MDR TB occurrence globally, and further illustrates the 53% of global MDR TB cases in the BRICS countries.
Psychological Effects
TB arises out of inequality, poverty, and constrained agency, which rarely includes a reasonable standard of care. As a result, stigmatization, isolation, depression, and despair can further reduce the state of health for those with TB.
Economic Impacts
Achieving the UN Sustainable development goals (SDGs) and the global target set at the 2018 UN meeting on TB will require approximately US$13 billion (WHO, 2023). 80% of spending on services is derived from domestic sources. Donor funding is heavily relied upon for both low- income and middle-income countries’ TB responses; TheGlobal Fund to Fight AIDS, TB, and Malaria is a key private donor source. $1billion was made available for research to develop new tools toward meeting the goal, a $1billion shortfall of the needs (WHO). Modern inequality is often structurally expressed through the idea of cost effectiveness in relation to TB treatment (Farmer, 2005).
International Infectious Disease Management Dynamics
The Social Determinants of Health provide the key explanation as to why TB remains a tenacious infection to this day. Ending the epidemic of TB is one of the United Nations (UN) Sustainable Development Goals (SDGs) for 2030 and progress has been made toward reducing the rate of TB globally. This goal is important, because the treatment regime requires multiple drugs for an extended period of time. People with constrained access or finances can have treatment consistency frequently disrupted or poor-quality drugs, which are ways drug resistant bacteria can develop. “Drug prices should not constitute the chief barrier to treatment for all TB patients” (Farmer, 2005, p. 133). Altogether, health is a fundamental human right along with other closely tied rights that are recognized in the Universal Declaration of Human Rights.
The ongoing TB epidemic has had some small successes in reducing incidence, at the same time greater treatment complexity has arisen with drug resistant, multidrug resistant, and extremely drug resistant strains emerging. As stated earlier, ending the TB epidemic by 2030 is one of the UN SDGs. As the target date grows closer and the will to fully support the endeavor by wealthier countries wanes, the effort largely relies on private donations. Fundamentally, MDR and XDR TB are ethical issues that we ignore at our own peril.
Case 6 Emergent Themes
- Highlights inequalities in the global system.
- Reinforces the importance of resource ethics and equity in treatment of those with infectious diseases.
- Underscores the need for greater investment in the development of new TB treatment tools.
- Emphasizes that TB is both a present and future threat, given that resistant strains are often both more transmissible, virulent, and far more difficult to treat.
Case 7: Polio: a Re-occurring Vaccine Preventable Childhood Agent
Another infectious disease that has affected humanity for thousands of years is polio. It is known as both a maimer and killer of children. Even though children are primarily affected, anyone not immunized can be impacted by polio. As an indicator of the ancient nature of polio, the Egyptian painting depicted below reveals a person with a wasted leg in Figure 12.

Poliomyelitis is caused by a highly infectious virus transmitted via the oral-fecal route, or sometimes contaminated water or food. The virus multiplies in the intestine producing rather insignificant symptoms of fever, fatigue, headache, vomiting, and muscle stiffness. The serious effects occur in a smaller proportion of the cases, where the infection crosses the blood brain barrier and nervous system is attacked, causing permanent paralysis within hours. If contracted, polio is not curable. On the other hand, polio can be easily prevented with vaccine.
Three types of polio virus occurred: serotype 1, serotype 2, and serotype 3. At present, only Type 1 remains endemic in the wild. Two vaccines have been developed, the oral polio vaccine (OPV) and inactivated polio vaccine (IPV) that is given as an injection. Dependent upon local circumstances, the vaccine can be used in different combinations for protecting populations (WHO).
Outbreak Description
Following the success of the eradication of Smallpox, the global decision to eradicate polio was made in 1988. This resolution gave rise to the Global Polio Eradication Initiative (GPEI), a public-private partnership for the monumental task engaging the collaborative strength of countries and communities. The effort to eliminate the scourge of polio represents decades of commitment to immunizations, surveillance, funding, and other activities worldwide (Badizadegan, K. et al, 2022). The world is now on the threshold of realizing this long sought after goal.
Progress in wild polio virus eradication has been certified by region, which means that virus transmission has been stopped in a specific group of neighboring countries. The WHO certified the Americas as polio-free in 1994; the Western Pacific region was certified in 2000; the European region certified in 2002; the South-East Asia region was certified in 2014; and Africa was certified in 2020. Only the Eastern Mediterranean Region remains working toward eradicating the wild polio virus. In 2024, the wild virus remains endemic in only two countries: Pakistan and Afghanistan. At this point in time, any case occurring is a public health emergency.
Virus eradication effort in Afghanistan and Pakistan has been elusive for several reasons. The key ongoing barrier is insecurity and active conflict in the area (Aylward et al., 2011). This factor has proven to have incidents of community trust erosion at multiple points. Another important regional factor contributing to ongoing viral transmission is the presence of underserved migratory populations (Aylward et al., 2011). The children in these mobile populations are often absent during vaccination efforts; additionally, they trek over long distances transmitting the virus. These challenging conditions have required innovation and adaptation (a key emergency management skill) for successful strategies toward eliminating wild polio transmission. The COVID 19 pandemic also prolonged the effort, as polio vaccine shortages occurred due to the massive COVID vaccine production efforts, and surveillance was interrupted. After numerous setbacks, the outlook in the region is optimistic (Aylward et al., 2011).
The bubble of good news dissipates with the evidence of a growing problem, outbreaks of “circulating vaccine-derived polio virus” (cVDPV). This setback arises from the oral polio vaccine, which uses a live attenuated virus (genetically weakened) and the altered virus is subsequently excreted via feces. The virus can transmit via the oral-fecal route in unsanitary conditions and where a population has reduced immunity due to sociopolitical reasons. The key issue is the likelihood of this weakened virus reverting to a virulent variety that affects the nervous system. The solution to these issues has been providing more vaccine to increase that population immunity. Enhanced surveillance is also necessary in the areas of cVDPV outbreaks. At the same time, populations are more mobile now than in the early part of the endeavor, and a new internation spread of virus is occurring. The endgame plan will require a parallel management of the vaccine produced outbreaks (Aylward et al., 2011).
A more nuanced obstacle involves insufficient financing to see the eradication work to the end, which has been an ever-present issue, often relying upon private donors. A similar issue occurred in the attempt to eradicate malaria around sixty years ago, where the efforts were 97% achieved, then the funding was lost for the endgame; resulting in more genetically robust mosquitoes that have spread malaria faster and further. The endgame funding and plan is critical to prevent a global resurgence of polio that would bring disability and death to many children.
Demographic Effects
As stated earlier, polio infections mostly affect young children, nevertheless, anyone not vaccinated can become ill with it. Permanent paralysis is seen in 1 out of 200 cases, and of those, 5-10% die when breathing muscles become affected (WHO).
The efforts given to eradicate the wild polio virus have successfully decreased cases by more than 99% since 1988. The estimated cases at the start of the eradication program, 1988, were 350,00 cases across more than 125 countries. In 2023, 12 cases occurred globally in the Eastern Mediterranean Region. By 2024, only 2 countries, Afghanistan and Pakistan, remain endemic with only 3 cases to date in 2024. Figure 13 below depicts this tremendous progress.
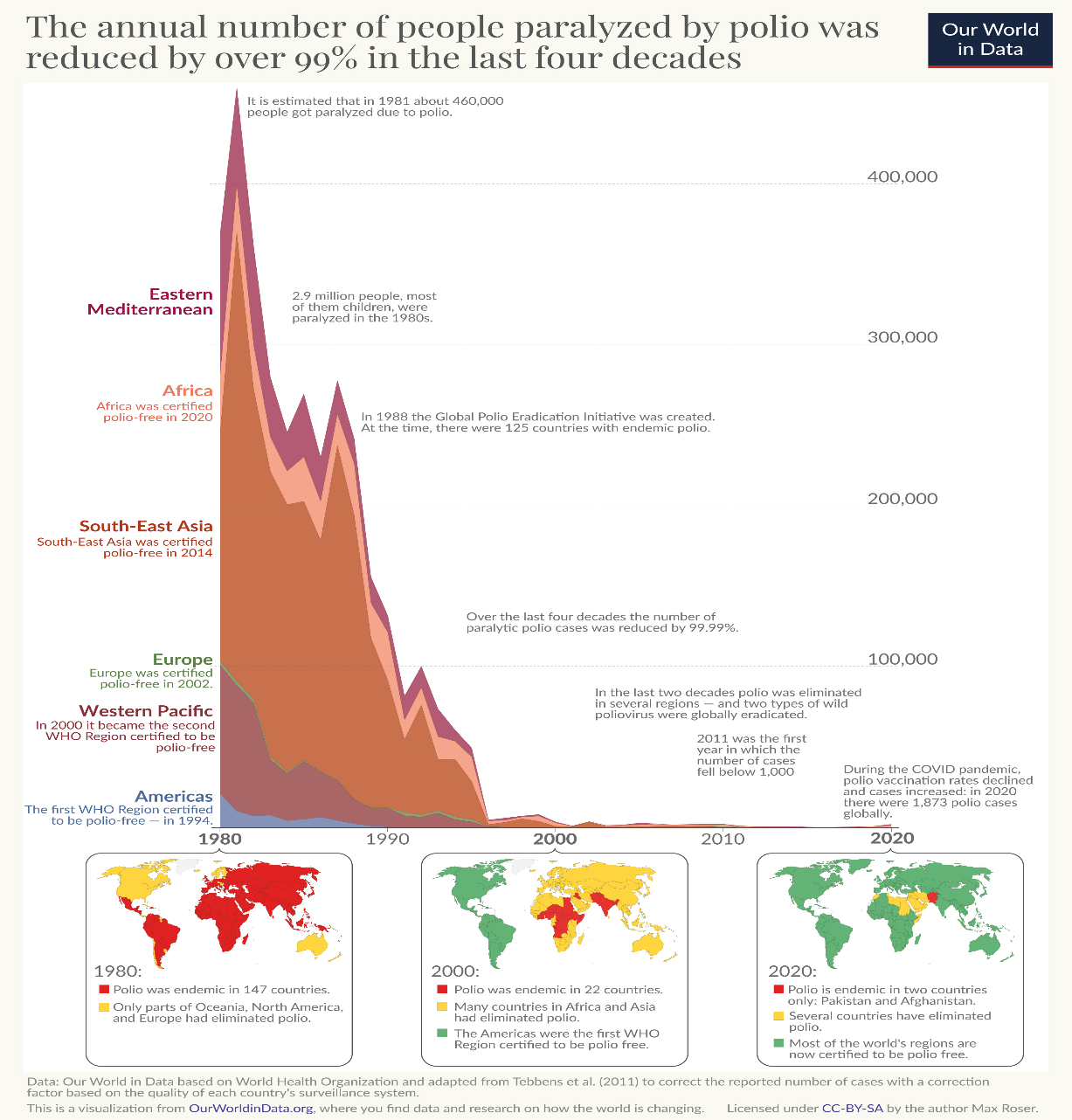
The good news of reduction in wild polio virus is dampened by the growing number of cVDPV cases. The incidence of vaccine derived polio virus rose from 366 to 1078 between 2019 and 2020 (Venkatesan, P., 2022)., and then went from 698 cases to 859 cases between 2021 and 2022 (MMWR, 2023). These increases have been attributed, in part, to the COVID19 pandemic causing interruptions in surveillance and vaccinations (Venkatesan, P., 2022). Moreover, some of these cases eventuated in flaccid paralysis. Further, this strain was found in 17 countries, even within some that transmission had been long eliminated (MMWR, 2023). The key anticipated problem with these rising numbers is that “in populations with low immunity, the virus can mutate and evolve” (Venkatesan, P., 2022) and a new version of polio could gain a foothold.
Psychological Effects
Children who survive the effects of a polio infection face a lifetime of limb weakness and mobility limitations, even full paralysis. Further, the emotional toll of isolation and disfigurement can be overwhelming. However, the increasingly positive outlook for eradication gives hope for a brighter future. To ensure the hopeful outcome, forward planning is essential for the end game success.
Economic Impacts
The money spent toward eradication programs is an overall money saver through the social benefits gained. The estimates involve a model-based process to characterize events that did not happen. The current work to monitor the disease occurrence and spread, continue immunizations and emergency response in the Eastern Mediterranean Region costs around US$ 7.5 billion. According to a study conducted for WHO (Badizadegan, K. et al, 2022; Klobucista, C., & Renwick, D., 2022), the return on this investment means lives saved and DALYs prevented, which economically yields ~US$38.70 for every dollar spent. Another way to look at the estimate is that the investment in the polio prevention programs yields an overall gain of US$ 289.2 billion.
International Infectious Disease Management Dynamics
As the end of wild polio may be nearing, any polio case is now managed as public health emergency. “As long as a single child remains infected, children in all countries are at risk of contracting polio” (WHO, 2024). Continued investment and improvement of surveillance quality is important for this future success.
Endgame planning is underway looking to a successful eradication of both wild polio and cVDPV. Active planning has included a “phased removal of the live oral vaccine”, “shift from sequential to parallel management of risks”, and a “framework for long term laboratory containment of all polioviruses” (Alyward et al., 2011, p.D84). If the global effort flounders and backs away from the endgame, it could result in a global resurgence of polio disease.
The leader of the smallpox eradication effort, D.A. Henderson, had words of caution toward the ideology of eradication (Henderson, 2009). The lessons learned he advocated for was “building and sustaining effective control programs that are adapted to the social and public health needs of each country” (Henderson, 2009, p. 303). The point being made was that eradication efforts can backfire with unique twists of fate, such as agent mutations, resurgence, and bioterrorism potential. Much more enduring is establishing trust, building community capacity and effective programs fitting the local context.
Case 7 Emergent Themes
- Reminds us that vaccines have a positive global impact.
- Underscores the threat of continued polio virus spread to areas where a population is insufficiently vaccinated (MMWR)
- Encourages us to build trust, which is essential in both eradication and infection control.
- Demonstrates that innovation and adaptation to obstacles has generated improvements.
- Highlights the need for improved, quality surveillance data.
- Insufficient financing prolongs the effort and gives opportunity to the virus.
- Any polio outbreak constitutes a public health emergency.
Case 8: 2013 Ebola Outbreak - Population and urban encroachment into Biodiverse zones, Zoonotic transmission, and poor risk management
Ebolavirus (EBOV) is a zoonotic viral hemorrhagic fever (VHF) disease. At least initially, it is passed on by wild animals such as bats, with moderate (droplet) transmission capability for those who come in close contact with bodily fluids (Jacob et al., 2020). This viral disease can ravage populations without access to adequate critical care and public health infrastructure.
Outbreak Description
The deadly, tragic West African Ebola virus disease (EVD) epidemic/pandemic of 2013-2016 was the largest in history (up to now) and a genuine global and regional public health crisis. Guinea, Liberia and Sierra Leone were the worst hit with at least 28,000 cases and 11,000 deaths. In stark contrast, the U.S.A. Ebola scare in the fall of 2014 had only 11 cases. Nine of the cases were med-evacuation imported West Africa-acquired cases, of those only 2 deaths. The other two infections were acquired by nurses infected because of poor protocols in a Texas hospital (McEntire 2019). The U.S. “outbreak” was primarily a political lighting rod and public health communication crisis, and a preview of the hysteria, misinformation, and conspiracy theories that novel outbreaks, including Covid-19, could foster. As cases turned up in the United States for the first time ever during the then-growing epidemic in West Africa, Ebola virus disease dominated much of the U.S. public airways.
Demographic Effects
This disaster was made possible by several variables, including tepid, late, and poorly resourced response by the international community and major institutions such as the World Health Organization (Al Jazeera, 2015). The Ebola outbreak in Zaire had as high as 60- 70% case fatality in remote African rural areas. Guinea, Liberia and Sierra Leone had over 28,000 cases and 11,000 deaths, and other West African countries were equally devastated by Ebola before it dissipated in 2016. Already evidenced in the social determinants of health, the most affected populations were the poorest, least educated, with little access to healthcare. However, Ebola was an agent that is not difficult to contain in developed countries with both modern hospitals and public health (Silver et al., 2015).
In the United States, the “Fearbola” (Blakey et al., 2015) domestic “crisis” began when a Liberian citizen visiting relatives in Texas was first misdiagnosed and turned away from a local hospital. The man later returned with worse symptoms, and he was finally diagnosed days after with Ebola virus disease. This individual was treated, but later died. His family accusing the facility of mistreating him because of his race, nationality, and poverty (Gambino, 2014). Within weeks, two of the nurses who treated him were also diagnosed with Ebola, including one who had flown while infected to Northeastern Ohio and back to Dallas (McEntire, 2019). Other infected Western healthcare workers and volunteers, who had been working in West Africa, were transported and treated in U.S., European, and other nations’ better equipped hospitals.
Psychological Effects
Ebola is physically and psychologically traumatic both because of the severe disease and mortality (and obvious fear of death) in infected individuals, and lingering “post-viral” symptoms (as seen also in “long COVID,” e.g.) neuro-psychological problems, including depression and cognitive impairment.
Over-taxed healthcare workers from West Africa and around the world (EBV infected 900, with over 500 dead) working in the “hot zone” have evidenced some post trauma effects. Severe psych-social quality of life effects on hard-hit communities are also observed. West African survivors went through psychosocial trauma because of feelings of shame about infecting others, and “stigmatization or blame from their communities.” Public health and medical institutions and personnel lost the trust of communities fearful of outsiders and an epidemic they little understood (Van Bortel et al., 2016).
In the U.S. system multiple factors, including political demagoguery, grandstanding, and ignorance, the 24-hour media infotainment “news” cycles, and hysteria-promoting internet rumors and memes create scientifically unwarranted, counter-productive, borderline hysterical reactions by the public and politicians alike. Although the two U.S. Texas nurses were the sole cases of Ebola, primarily due to very lax infectious disease control biosecurity measures at Texas Presbyterian, there was tremendous political pressure to ban travel and quarantine travelers who had been to West African countries undergoing the epidemic. While public health officials at the Centers for Disease Control and Prevention (CDC) and the Obama administration opposed federal travel bans as being ineffective and counterproductive, a number of governor-imposed quarantine and flight restrictions. In an election year (2014), airlines, schools, businesses, and streets were needlessly closed and fumigated based on even vague associations or rumors of who or what might have been exposed to Ebola. The entire contents of Liberian Thomas Duncan’s family’s apartment in Dallas were burned. Nevertheless, in some cases in Ohio and Texas, these measures averted more costly voluntary disruptions, by, for instance, helping assure the parents of school children that they could send their offspring to school, or workers that they would be safe from Ebola. Some states declared travel bans and quarantines for recent travelers from Ebola-stricken countries, including healthcare workers, and there was considerable domestic stigmatization and discrimination leveled against West Africans or anyone associated with recent travel to the region (Bioethics Commission 2015).
Economic Impacts
Response to the first cases took weeks by WHO, and earlier intervention would have caught the almost exponential growth in Ebola cases and blossomed into unmanageable proportions. However, the WHO had also been subject to budget cuts in preceding years (by its member nations, especially the United States Congress) in the very programs dedicated to rapid infectious disease rapid response; its overall budget had been cut by nearly $600 million in 2011. In addition, WHO had also shifted in the years before institutional priorities from infectious diseases to non-infectious ones (cancer, diabetes, heart disease) that reduced ID staffing to a third of what it had been. Critically, the entire WHO budget (for the world) is only a third of the U.S. CDC (Ingham & Zingg, 2015).
International Infectious Disease Management Dynamics
The West Africa-originated Ebola outbreak was a significant epidemic that was mismanaged in many regards. Early and dire warnings were made from Medicins Sans Frontieres (MSF or Doctors without Borders) and other already-overstretched non-governmental organizations that had already been operating in the region and provided a critical early role that likely prevented a far worse outcome (Caleo, 2021). The international response was slow with limited resources. The high population nation of Nigeria barely managed to contain their outbreak from becoming a runaway disaster (Althaus, 2015).
Meanwhile, the WHO was accused of delays and significant mistakes, especially in the early part of the crisis, which likely worsened the outbreak (Al Jazeera 2015). Health systems in affected developing countries were overwhelmed and disrupted by Ebola, and their capacities were significantly reduced for years, during and after (Van Bortel et al., 2016). All of these factors, and the complacency of the developed world – unaccustomed to having to worry about most infectious diseases of the poor – contributed to a colossal failure to prepare for acute infectious disease risks. In an interconnected, “globalized” and urbanized world, infectious diseases both confound international borders and remain a significant Third World disaster risk (Keita et al., 2024). Ebola foreshadowed the many potential problems that will arise in the inevitable case of future, more serious outbreaks or pandemics, including future biological terrorism (McEntire, 2019).
Case 8 Emergent Themes
- Highlighted the lack of global preparedness and response capabilities.
- Challenged global trust in the United Nations WHO based public health response system.
- Demonstrated why even highly deadly diseases can present only a mild threat to wealthier, developed countries with competent public health systems, if transmissibility is only moderate – by intimate droplet and close proximity, and not by aerosols
- Verified the important need of ongoing public health surveillance and response infrastructure within less developed nations.
- Demonstrated the need for clear and authoritative communications to the public.
- Revealed the need for integrating psycho-social considerations into emergency planning for response and recovery at both the individual worker and community levels.
Case 9: 2019-2023 COVID-19 – Catastrophic Novel Viral Pandemic
The poignant foreshadowing by the 2003 SARS-CoV1 outbreak gave way to the horrifying realities of the 2019-2023 COVID-19/SARS-CoV2 pandemic. Many of the attributes and lessons are still being studied from this consequential novel coronavirus. Recently, for example, a link has been established between climate change-related wildfires, respiratory infections like Covid-19, and premature deaths. The studies show small particulate pollution from wildfires in the Western United States increased Covid-19 death in Oregon, Washington, and California in 2020 (Zhou et al., 2021).
Outbreak Description
The novel SARS-CoV-2 coronavirus responsible for severe acute respiratory syndrome Covid-19 disease first emerged in Wuhan, China in November 2019. While the exact origins of the virus remain uncertain and even disputed, it caused the largest acute pandemic in 100 years, since the Great Influenza Pandemic of 1918-1919. But the pandemic did damage to most of the world, arrived in waves at different times and places, and was affected by many unique and surprising variables (O’Sullivan & Ramsay, 2020).
The Covid-19 pandemic’s transmissibility and death rates are still being discovered by retrospective studies. Unlike its cousins, SARS-1 and MERS, most people had mild cases of Covid-19. But because a large percent of the world became infected, the numbers of hospitalized and dead were also very large and overwhelming to already overstretched medical systems. In the United States, people of color were particularly susceptible to severe disease – overwhelming particular communities more than others.
The high transmissibility of Covid-19 disease was related in part to progressive mutations in the viral strains that emerged from the original version – made more so because so many people around the world became infected, often multiple times. That is, infection / transmissibility increased over time. As with other viral aerosolizable respiratory diseases, age, pre-existing medical conditions, social vulnerability, and access to healthcare, genetics, and luck played into the severity of illness (Xiong et al., 2020). Also important was people’s vaccination rates, use of non-pharmaceutical counter measures such as masks, distancing, isolation, etc.
Demographic Effects
Worldwide, Covid-19 infected over 700 million and caused at least 7 million deaths – including 110 million-plus Americans infected, and 1.2 million dead (WHO, 2024). Beyond China, among the locations hit hardest in 2020 were northern Italy, Spain, and in the U.S. state of Washington, the New York City region, Detroit/Michigan and southern Louisiana.
Covid highlighted the economic and public health system disparities, and overall social determinants of health around the world. As noted above, after vaccines were introduced and mass-produced, developed nations had rapid uptake. But by 2023 more than thirty percent of the world was still unvaccinated, including 77% of individuals in low-income countries (Yang et al., 2022).
Psychological Effects
Covid-19’s psychological damage still reverberates around the world – both for trauma-related mental health reasons and physiological ones associated with so-called long Covid. Studies showed the COVID-19 pandemic psychologically affected everyone in the world, magnified by various public health restrictions (shut-downs, distancing, etc.) and other long-term repercussions, but also from the fear of illness and death, and actual illness, hospitalization, and death of those around them. Compared to before the pandemic, the World Health Organization (WHO) estimated the Covid-related anxiety and depression prevalence increased by 25% globally (Kupcova et al., 2023)
Another unfortunate result of the pandemic’s early uncertainties, often magnified by social media and public officials, were rumors and misinformation circulated as conspiracy theories, suggesting that the MRNA vaccines did not work or were dangerous (neither of which was true), masks were useless -- or a violation of civil liberties, Covid-19 was no worse than a cold, etc. And rampant and persistent rumors about various ineffective, untested and often dangerous treatments like hydroxychloroquine insisted they were being suppressed by drug companies or authorities seeking to get rich. For instance, hydroxychloroquine (HCQ) use was associated with an 11% increase in the mortality rate in a “meta-analysis of randomized trials (17,000 HCQ-related deaths estimated among hospitalized patients in six countries)” (Pradelle et al., 2024). And the horse de-wormer ivermectin, popular in vaccine-denial circles, was confirmed to be ineffective in a randomized double-blind placebo-controlled platform trial (Naggie et al., 2023). Such risks are magnified given social media/internet rumors and misinformation in the absence of scientifically supported treatments – as can happen with any novel virus outbreaks.
Economic Impacts
The pandemic and concurrent “compound disaster” events demonstrate that infectious disease disasters can be among the most damaging economic, political, and societal security threats, in large measure because they can undermine the structure of international and domestic trade and commerce. Estimates vary, but Covid-19 caused most of the world to fall into economic recession. By March 2020 the outbreak had already caused market value losses of an estimated $4 trillion, particularly catastrophic among the travel and hospitality sectors, as well as those reliant on international (vs. domestic) supply chains (Sullivan & Cowan, 2020). As of 2021 global economic output was over 4 percent down from pre-pandemic trends, and global trade had been significantly disrupted (since mostly recovered). Governmental responses at a policy level led to increases in government deficits and debt, as nations (where possible) spent money to soften the blows of unemployment, increased costs, and trade loss. And the combination of factors at least temporarily substantially increased inflation, food and other goods’ prices, and overall cost of living around the world (Martin et al., 2023).
In the United States, for example, COVID-19 in 2020 temporarily caused the largest economic downturn since the Great Depression. “GDP fell at a 32.9% annualized rate, the deepest decline since records began back in 1947… and 30.2 million Americans were receiving unemployment checks in the week ending July 11” (Mutikani, 2020)
One study estimated the rapid development and deployment of (especially the mRNA) vaccines in 2021 alone saved the world 150 million cases, from 600,000 – 2 million deaths, and over $150 billion in averted infections. Rough figures estimated that globally 6.3 billion COVID-19 vaccine doses were given by October 2021, and by the end of 2022 doubled to almost $13 billion doses. (Yang et al., 2022).
International Infectious Disease Management Dynamics
Covid-19 demonstrated many shortcomings in the global medical and public health systems, where most healthcare systems (with some notable exceptions outlined in this chapter) were unaccustomed to such large-scale infectious disease outbreaks from novel viruses (something already foreshadowed with MERS, SARS-1, HIV/AIDS, and Ebola before it), let alone genuine acute pandemics. Different countries often took diametrically opposed positions, dealing with the outbreak, ranging from draconian closures and distancing (Italy, e.g., where at various points no one was allowed on the streets for a time) to lax distancing policies (such as Denmark’s) (Andersen et al., 2022). The initial lack of vaccines or antiviral treatments required non- pharmaceutical countermeasures such as social distancing, isolation of the sick, quarantine of those possibly infected, and business and trade closures that took a heavy toll. International shortages and hoarding of N95 respirator masks (including among healthcare providing institutions), alcohol hand sanitizer, and even surface cleaning products quickly developed.
Public health and medical systems were damaged in short- and long-term ways. Previous privatization and staff cuts begun many years before purported to maintain quality of care, but Covid-19 finally overwhelmed an already brittle system. Related illness and death among healthcare workers and their families, psychological burnout, and fears of job-related infection for workers and their families, collided with already critical staffing shortages. Studies show that the nursing workforce in particular was associated “inversely and significantly correlates with COVID-19 [case-fatality rates]; that that observation was independent of other confounding effects, and perhaps not surprising that nursing professionals per patient was not just the largest, but the only factor identified that significantly reduced COVID-19 CFR, when controlled for health expenditure, median age, physician density, and urbanization for analyzing their individual predicting effects on COVID-19 CFR” (You & Donnelly, 2024).
The pandemic also showed how little most people, even much of the medical community, knew about severe viral infectious disease (ID) epidemics up to that point. In wealthy nations, most pre-Covid were from chronic diseases – cancer, respiratory, cardiac and other metabolic diseases, etc. Pandemic disease is notable because there is no one individual – or even institution – that holistically grasps the complex scientific, medical, social, psychological/behavioral, economic, and political variables and systems involved. (O’Sullivan & Ramsay, 2021).
Finally, at least 14 species of bats (known to be the origin species hosts that carry coronaviruses, and harbor them without illness) carry a minimum of 4000 other coronaviruses that might have the potential for zoonotic human infection, given the right genetic changes in the wild (Ruiz-Aravena et al. 2021). Troubling intersectionalities exist among the bats as coronavirus hosts, Covid-19 and its cousins MERS and the 2003 SARS-1, global climate crisis, and other human environmental insults. Habitat disruption and human hunting of bats and other mammals that could be intermediary hosts contributed to the “spillover” exposures in at least both SARS viruses (Covid-19 included). Moreover, early evidence now suggests that evolution of the MERS-CoV and SARS-2 Covid-19 coronaviruses could enable them to “recombine” in living cells, potentially creating a new, superbug version of both (Wang et al. 2023).
Case 9 Emergent Themes
- Exemplified how coronaviruses now represent a new pandemic threat alongside influenza A.
- Evidenced many interdependencies and interactions in risk; including globalized trade, travel, urbanization, climate change, and vector/host exposure, and genetic recombination potential for future coronavirus superbugs.
- Exposed resource limitations, both in public health and clinical, that contributed to diminished outcomes for healthcare workers and patients.
- Established the world is less prepared for the next pandemic.
- Validated the need for continued accelerated investments for public health pandemic response and recovery.
- Revealed limited medical surge capacity / “just in time” health care systems overwhelmed.
- Validated that global infectious disease hazards and human susceptibility to respiratory diseases will be increasingly affected by climate crisis.
- Reinforced the need for continued development investment in vaccines.
- Reinforced that clear and authoritative public education is necessary, with countermeasures against misinformation.
- Revealed novel sources of biological risk.
Case 10: Dengue and Zika and Climate Change Interactions: VBD Expansions
Vector-borne diseases (VBDs) are carried by a variety of organisms, including mosquitoes, ticks, bugs, flies, snails, fleas, etc., and represent more than 17% of all human infectious diseases, causing more than 700,000 deaths annually. The actual pathogenic diseases these “delivery” vectors carry can be caused by a range of parasites, bacteria or viruses. The most prominent is mosquito-borne parasitic malaria.
Dengue (aka dengue fever and ‘breakbone fever’) is a “re-emerging” infectious disease, which is underreported, rapidly expanding and societally damaging. The virus is carried mainly by the Aedes egypti mosquito. The RNA virus has four variations/serotypes that can cause disease; prior infection with one type is a risk factor for later infection at a more serious level. Milder symptoms include fever, rash, nausea, and muscle and joint pain, while severe dengue brings complications that can result in internal bleeding, shock, and death.
Another important mosquito-borne vector-borne disease is Zika virus, originating in East Africa and Asia, and is a Western Hemisphere public health emergency. As with dengue, no treatment or vaccine is available currently; similarly, most virus infections are mild or unnoticed. Three characteristics have made the infection dangerous. First, Zika can cause severe disease, including Guillain-Barre syndrome paralysis in adults. Second, it has been linked in somewhat rare instances to severe birth defects, including neonatal microcephaly brain disabilities in babies born to infected mothers, and because of it, Zika public health measures principally focus on preventing infection in pregnant women, in particular (Plourde 2016).Third, a troubling “antibody-dependent disease enhancement” (i.e., severe immune responses when previous dengue infections meet subsequent Zika infections, and visa-versa) (Katzelnick et al., 2021). “Despite growing knowledge, questions remain regarding the virus’s vectors and reservoirs, pathogenesis, genetic diversity, and potential synergistic effects of co-infection with other circulating viruses. These questions highlight the need for research to optimize surveillance, patient management, and public health intervention” (Plourde 2016).
Outbreak Description
The aedis egypti was once controlled and almost eliminated, when funding toward the effort collapsed. The hardiest remaining mosquitoes began to replicate exponentially. Figure 14 below illustrates the widening range of the mosquito infestation between 1970 and 2002. The global warming crisis is now accelerating this vector expansion further north and south of the tropics. Further, the genetically similar dengue and Zika virus epidemics continue to interact and spread in overlapping territory across the globe (Katzelnick et al. 2021). The dengue virus has progressively made its way from being a neglected tropical disease to becoming a 20-year-plus long pandemic.
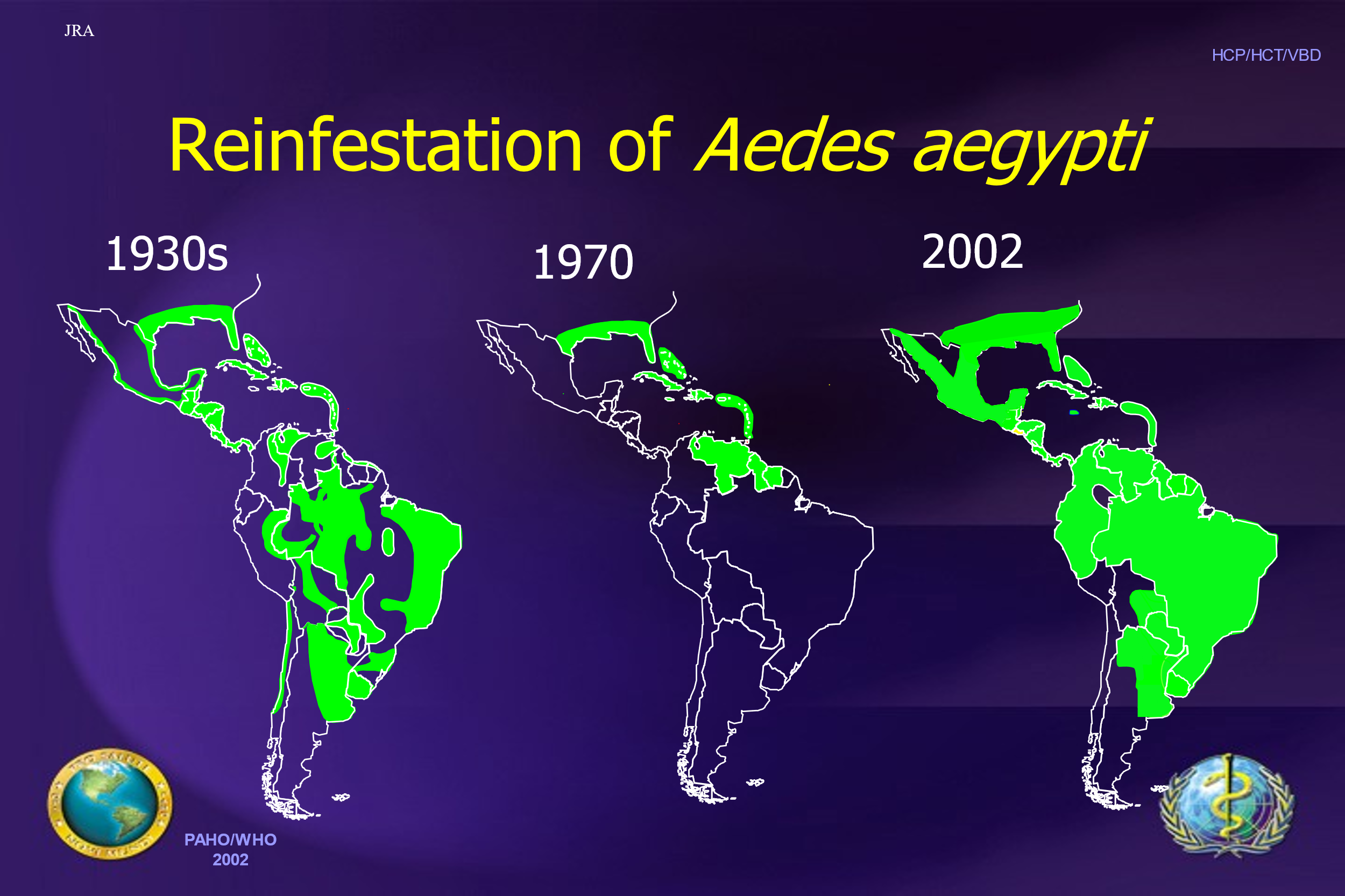
The broad geographic scope dengue currently covers deems it a pandemic. The tropical parts of the planet are now expansively becoming endemic. The current global dengue risk is depicted below in Figure 15. Around 4 billion people -- almost half of the world's population – live in dengue risk areas, and travelers to these areas are also vulnerable to infection, primarily in South Asia, Southeast Asia and the Caribbean.
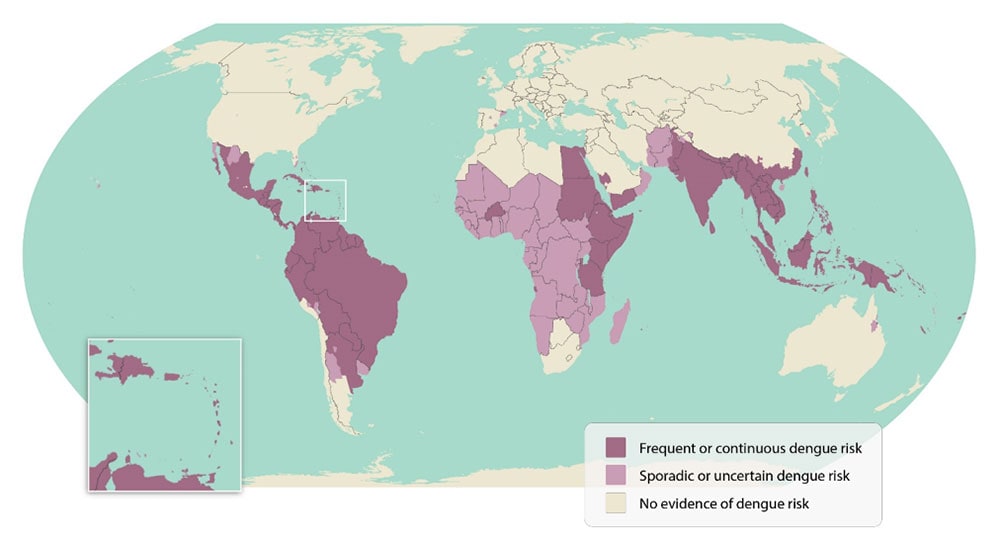
Dengue has growing importance because: 1) the climate-magnified 8-fold increase of reported cases over the first 20 years of the 21st century, and 2) it is often a major debilitating disease that contributes greatly to poverty.
Demographic Effects
Dengue is now endemic in over 100 countries with more than 40% of the global population living in these areas. Each year an estimated 390 million infections (96 million symptomatic) and 21,000 deaths every year (Zeng, 2021) are attributed to dengue. Further, dengue modeling studies suggest that dengue is greatly underreported.
Dengue is one of the many dramatic social vulnerability variables for urban, peri-urban and rural communities with poor living conditions – particularly those lacking access to sufficient minimal nutrition and calories (which creates weakened immune systems), housing, safe drinking water, and sanitation. These are people hit the hardest by vector borne pathogens, which in turn worsens their poverty as disease and disability can severely effect family incomes by preventing people from working, having to pay for expensive medical care, and other forms of exacerbated financial hardship (WHO, 2017).
Psychological Effects
Although dengue results in a large percent of mild cases for people initially infected with one of the four strains, people who are so unfortunate as to contract a 2nd, 3rd, or 4th infection have a significantly different experience. Such a follow-on infection with a different viral strain can experience, excruciating pain and suffering, even they don’t die. The memory of the pain and suffering endures and can be traumatic. Dengue is particularly feared among poor people in slums with poor mosquito control, public health, and economic resilience (Daude, 2017).
Economic Impacts
Studies from multiple hard-hit countries show an average dengue episode lasts 15 days (of lost work) and costs an average US $514 (in some countries fully half of yearly poverty incomes) for mild to moderate stay-at-home illnesses, and 19 days of lost work hospitalized patients who don’t die, averaging US $1491. No income coupled with hospital costs can be a staggering loss for families already suffering from increasing food prices exacerbated by climate- and war-damaged global crop outputs. Controlling VBDs in general, therefore, plays a critical role in reducing poverty, disability, and improving economic development in countries whose income levels may be depressed by as much as 60% less than similar countries without such plagues (WHO, 2017).
International Infectious Disease Management Dynamics
Because there are no viable vaccines or treatments, mosquito- and other viral VBDs like yellow fever, West Nile disease, dengue, and Zika are only minimized by control of breeding grounds (draining swamps and other standing water, chemical insecticides, etc.) and careful monitoring of cases. But given the tremendous resources involved in such efforts at a time of global reductions of public health funding and increasing competition from other expanding public health burdens (such as the Covid-19 pandemic, e.g.). And as the warming accelerates (as it has been the last 20 years) the disease risk variables will require more resource triage and adaptation strategies – including greater focus on vaccines. Promising dengue vaccines are in the works, but they would be years away from rollout, regardless.
Case 10 Emergent Themes
- Demonstrates a worsening public health effects of climate change; it is the fastest spreading viral mosquito-borne disease.
- Interacts with other emerging VBDs, such as Zika virus.
- Calls attention to the resource challenges surrounding mosquito control.
- Reinforces the need for vaccine development.
- Underscores the economic burden of dengue and other mosquito-born diseases’ effect on DALYs to societies.
- Emphasizes the acute need for public health resources: VBD control specialists, strategic health policies, and greater surveillance data.
Discussion
Humanity faces countless biological risks in the 21st century, and the devastating Covid-19 pandemic (SARS-CoV-2) may have been a mere warning shot to get ready. As highlighted above, the natural hazards risks range from growing reemerging infectious diseases such as dengue and tuberculosis, to alarming emerging IDs with actual or pandemic potential from the coronavirus and influenza viral families. All of which are exacerbated by climate crisis-related disaster variables, global trade, travel, immigration, refugees, and human incursion into wild environments that harbor potential microbial monsters. Unquestionably, as biological technology advances in leaps and bounds, the threat of old or newly engineered bioweapons from malevolent state and non-state actors also looms.
We found that four overarching commonalities became clear from our review of the 10 case studies mentioned in this chapter. These fundamental categories the key themes fell under are: 1) Learning Reinforcement Themes, 2) Validating Themes for Continued Investment, 3) Planning Improvement Themes, and 4) Policy Implication Themes. Presented below is Table 2 with the categorized emergent themes and sub-themes and the cases they were most obviously evidenced in, though the reader will note, and we acknowledge, that most of the cases fell into most of these subthemes at some level.
|
Key Emergent Themes
|
|
|---|---|
|
Learning Reinforcement Themes |
Cases evidenced in=11 |
|
Prompted learning and improvement(s) |
1, 2, 7 |
|
Confirmed that investment in preparedness &/or vaccine pays off |
3, 7 |
|
Reinforced hygiene awareness & prevention strategies, including vaccine |
2, 7, 9 |
|
Reinforced that early, clear, straightforward, and authoritative public information is necessary to minimize misinformation. |
3, 7, 8 |
|
Validating Themes for Continued Investment |
Cases evidenced in=23 |
|
Validated the need for trust building |
3, 7, 8, 9 |
|
Validated the need for greater surveillance data |
3, 6, 7, 8, 9, 10 |
|
Validated the need for continued investments for response & recovery |
1, 3, 4, 7, 8, 9, 10 |
|
Validated the need for investments in development: vaccines, sanitation, clean water, & treatment tools |
2, 3, 6, 8, 9, 10 |
|
Planning Improvement Themes |
Cases evidenced in=18 |
|
Revealed preparedness weaknesses or poor risk recognition |
1, 5, 8, 9, 10 |
|
Substantiated rapid social destabilization |
1, 2, 4, 5, 9 |
|
Damaged long term mental health of the population affected |
2, 7, 8, 9 |
|
Revealed limited medical surge capacity / health care systems overwhelmed |
2, 3, 4, 9 |
|
Policy Implication Themes |
Cases evidenced in |
|
Evidenced scarce resources / resource ethics tests |
6, 7, 9, 10 |
|
Highlighted inequalities |
2, 6, 8, 9, 10 |
|
Accumulated economic damage –tourist dependence, reduced DALYs |
1, 2, 4, 8, 9,10 |
|
Detected confusion or controversy with existing policies |
3, 5, 9 |
|
Demonstrated complex interdependencies in outbreak &/or coordination |
1, 2, 3, 4, 5, 6, 7, 8, 9, 10 |
|
Substantiated a growing treatment resistance / control difficulty |
6, 10 |
|
Revealed novel sources of bio risk: superspreader, research laboratory leakage, biotechnology, and conditions producing resistance. |
4, 5, 6, 7, 9 |
Despite the sobering conclusions about rising risk and insufficient preparedness, some of the cases also reveal instances of systems-level learning with steps toward continual improvement. This learning and growth cycle is one that influences adaptation but requires more intentional focus of effort to be ready for future outbreaks. Of critical importance, the obligation of continued investment was repeatedly demonstrated as vital for human survival. The specific resource and funding needs include further development of global sanitation, clean water access, vaccines and treatment tools, surveillance everywhere – from cities to remote remaining wild areas – for timely data access, and health emergency response and recovery capacity.
The overwhelming lesson here, aside from the necessity of far greater understanding of these complex, synergistic risk variables, is the absolute need for far greater investments in preparedness and planning – all of which requires resources and informed public policy. Specific planning needs identified across cases include building medical surge capacity, building greater social resilience to withstand shocks of outbreaks, integrating trauma informed concepts into planning for long-term mental health benefits, and widening preparedness planning to include the range of biological disrupters so that response and recovery will have a better footing during an emergency.
In the policy realm, short-sighted economic and social justice structural problems magnify not only inequalities but are directly linked to negative overall societal and global outcomes – because, as the world now knows from Covid-19, infectious diseases know no boundaries. The lack of Covid-19 vaccines in the developing world, for instance, had a direct impact on the evolution of new, more transmissible strains emerging to sweep the global. Secondly, new scientific knowledge involving pathogen resistance to treatment or novel sources of bio risk requires investment for translational research actions and policy formulation to generate appropriate future guidance to timely response, adaptation, and recovery.
Significantly, all the cases presented in this chapter demonstrated forms of interdependencies and interactions either in the pathogen outbreak itself, in the management of the outbreak, or both. Often new, evolving, and uncertain conditions can cause confusion or controversy with existing policies or plans; thus, the two emergent themes also appear interrelated. The interactions between the social, built, physical, and microbial environments produce an ever-changing situation in an outbreak. As a result, many new unknowns can thwart the well laid plans of yesterday.
Understanding Interdependencies
The dynamic forces at play suggest that a systems-thinking approach can inform a clearer understanding of bio threats. When a bio-hazard event disrupts a system, the overarching goal is to convey the known risk to produce awareness and activation across the system. This shared meaning creates the unity necessary to support collective adaptive action, which is a cumulative process bringing the system back to a stable state (Comfort, 2007).
The complex interrelationships of the microbial and human worlds imply seeing that the borderless bio risks and the many organizational layers of response (local, district or state, national, international, and regional, government and non-government) form a system of systems. The systems can be visualized as a “set of networks that facilitate the exchange of incoming and outgoing information through a series of analytic activities that support the system decision process” (Comfort, 2007, p. 195) and creative problem solving given a shared meaning or common goal. The opportunity presented here is to understand that this dynamic global interorganizational system of systems can be influenced at all levels to optimize interactions using four key decision points, as characterized by Comfort (2007, p. 189).
- Detection of risk,
- Recognition and interpretation of risk for the situation,
- Communication flow of the risk across the system(s), and
- Self-organization and adaptive mobilization of collective community response.
In emergency management, policy and decision making in a dynamic interorganizational response system requires cognition about the bio risk situation as the activating point of response.
Cognition is a process of continuing inquiry, building on prior knowledge of the region at risk and integrating incoming information on changing conditions and system performance into a current assessment of vulnerability of the community (Comfort, 2007, p. 193). The intent of this chapter is to build prior bio risk knowledge for emergency managers, so that the risk signals become recognized earlier in an unfolding event.
The case studies presented in this chapter were rich in information and content, with the endeavor to reframe the events for emergency management relevance. Global and domestic public health and emergency management have always responded to infectious disease crises, sometime better than others, as discussed above. But only in the late 2010s, just before Covid-19, has there been a systematic effort to conceptually integrate the two under public health emergency management (PHEM) as a sub-discipline (Rose et al., 2017). This chapter seeks to contribute to that nascent literature. And part of that agenda necessitates finding ways to help current and future professionals learn at least a consumption-level literacy in global biosecurity, surveillance, preparedness, and PHEM response in the context of complex adaptive systems (O’Sullivan 2023).
The beginning point in reframing content for a specific discipline is to conduct case studies. The case study design, as expected, has intrinsic limitations. Yet, case studies are an essential starting point for beginning to integrate the knowledge of biosecurity risks for the emergency management field and discovering the important foundational constructs for cognition of the threat to occur earlier in an unfolding event.
Conclusion
The Covid-19 pandemic was clearly the domain of both public health and clinical medicine, but it drove home the increasingly critical role emergency management plays as a more recent stakeholder in disasters of this type. The goal of this chapter is to present the fundamental concepts of “the trade,” including the unique and highly complex qualities of naturally occurring and unnatural/human-caused ID hazards and disasters. A literature review provided the background and common terminology to become familiar with for health emergencies. Foundational population health concepts and some important historical cases were covered.
The methodology established the 10 key roots of outbreaks, which informed the categories of cases selected. All cases selected occurred in the 21st century and each represented one of the key outbreak roots. The 10 cases included: 2004 COV1, 2010 Haiti Cholera outbreak, 2009 H1N1 pandemic, 2012 MERS outbreak, 2001 Anthrax bio-terrorism cases, the 30-year TB outbreak in Russia, the nearing eradication of polio, the 2013 Ebola outbreak, 2019-2023 Covid19 pandemic, and the 20 year long pandemic of Dengue fever. From each of the cases discussed, themes emerged, which shared some commonalities with other cases. The characteristics of the commonalities include: 1). Learning Reinforcement Themes, 2). Validating Themes for Continued Investment, 3). Planning Improvement Themes, and 4). Policy Implication Themes.
These themes and findings have significant relevance for emergency management professionals, particularly in the continual improvement cycles of planning and after action, as well as policy implications. The findings emphasize that the core competencies needed in emergency management now require biological risk training. As emergency managers become better versed in the risk landscape of biological threats, that expertise will help mitigate future local to global infectious disease risk management failures among fragile complex systems.
References
Adalja, A.A., Toner, E., & Inglesby, T.V. (2015). Clinical management of potential bioterrorism-related. New England Journal of Medicine, 372(10), 954-962.
Administration for Strategic Preparedness and Response (ASPR). (2024). Medical Surge Capacity and Capabilities (MSCC) Handbook. U.S. Department of Health and Human Services. https://aspr.hhs.gov/HealthCareReadiness/guidance/MSCC/Pages/default.aspx
Ahmadzadeh, J., Mobaraki, K., Mousavi, S. J., Aghazadeh-Attari, J., Mirza-Aghazadeh-Attari,M., & Mohebbi, I. (2020). The risk factors associated with MERS-CoV patient fatality: A global survey. Diagnostic microbiology and infectious disease, 96(3), 114876.
Althaus, C. L., Low, N., Musa, E. O., Shuaib, F., & Gsteiger, S. (2015). Ebola virus diseaseoutbreak in Nigeria: transmission dynamics and rapid control. Epidemics, 11, 80-84.
Andersen, P. T., Loncarevic, N., Damgaard, M. B., Jacobsen, M. W., Bassioni-Stamenic, F., & Eklund Karlsson, L. (2022). Public health, surveillance policies and actions to prevent community spread of COVID-19 in Denmark, Serbia and Sweden. Scandinavian Journal of Public Health, 50(6), 711-729. Sage.
Aylward, B. &a Tangermann, R. (2011). The Global Polio Eradication Initiative: Lessons Learned and Prospects for Success. Vaccine 29(4).
Badizadegan, K., Kalkowska, D.K., Thompson, K.M. (2022). Polio by the Numbers: a Global Perspective. Journal of Infectious Disease226.
Barber. L.M., Schleier, J.J., and Peterson, R.K. (2010). Economic Cost Analysis of West NileVirus Outbreak, Sacramento County, California, USA, 2005. Emerging Infectious Disease, 16 (3), 480-486.
Barry, J. M. (2004). The site of origin of the 1918 influenza pandemic and its public health implications. Journal of Translational medicine, 2(1), 3.
Beck, U. (1992 Risk Society: Towards a New Modernity. Sage.
Bell D.M., Weisfuse, I.B., Hernandez-Avila M, del Rio C., Bustamante X, & Rodier G. (2009). Pandemic influenza as 21st century urban public health crisis. Emerging Infectious Disease. DOI: 10.3201/eid1512.091232
Berche, P. (2023). Gain-of-function and origin of Covid19. La Presse Médicale, 52(1), 104167.
Berger, K. M. (2021). Technological Advances that Test the Dual-Use Research of Concern Model. In Applied Biosecurity: Global Health, Biodefense, and Developing Technologies (pp. 133-160). Cham: Springer International Publishing.
Blakey, S.M., Reuman, L., Jacoby, R. J., & Abramowitz, J. S. (2015). Tracing “Fearbola”: psychological predictors of anxious responding to the threat of ebola. Cognitive Therapy and Research, 39, 816-825.
Boischio, A., Sanchez, A., Orosz,Z., and Charron, D. (2009). Health and Sustainable Development: Challenges and Opportunities of Ecosystem Approaches in Prevention and Control of Dengue and Chagas Disease. Cad. Saude Publica, S149-S154.
Bower, W. A., Hendricks, K. A., Vieira, A. R., Traxler, R. M., Weiner, Z., Lynfield, R., & Hoffmaster, A. (2022). What is anthrax? Pathogens, 11(6), 690.
Bray, R.S. (2000). Armies of Pestilence: The Impact of Disease on History. James Clark & Co Ltd.
Brown, G.G., & Cox, Jr, L.A. (2011). How probabilistic risk assessment can mislead terrorism risk analysts. Risk Analysis: An International Journal, 31(2), 196-204.
Brown, T. (2011). Scientists Asked Not to Publish H5N1 Flu Research Details,” Medscape Medical News (December 22, 2011). Accessed at http://www.medscape.com/viewarticle/755953
Brown, T. (2012). “H5N1 Influenza Researchers Meet, Trying to Break Impasse”. Medscape Medical News (February 17, 2012a). Accessed at http://www.medscape.com/viewarticle/758880
Brüssow, H. (2023). Viral infections at the animal–human interface—Learning lessons from the SARS‐CoV‐2 pandemic. Microbial Biotechnology, 16(7), 1397-1411. Wiley Online.
Burkle, F.M. & Greenough, P.G. (2008). Impact of Public Health Emergencies on Modern Disaster Taxonomy, Planning, and Response. Disaster Medicine and Public Health Preparedness 2(4).
Burns, W. J. (2007). Risk perception: A review. Report, USC CREATE Center. https://create.usc.edu/wp-content/uploads/2023/05/07-001.pdf
Butler, A.S., Panzer, A.M., & Goldfrank, L.R. (2003). Preparing for the Psychological Consequences of Terrorism: A Public Health Strategy. National Academies of Science.
Bykov, I., Dyachenko, O., Ratmanov, P., Liu, H., Liang, L., & Wu, Q. (2022). Factors Contributing to the High Prevalence of Multi-drug resistance/ Rifamicin-resistance in Patients with Tuberculosis: An Epidemiological Cross Sectional and Qualitative Study from Khaboravsk Krai Region of Russia. BMC Infectious Diseases 22(612). https://doi.org/10.1186/s12879-022-07598-7
Caleo, G. (2021). Epidemiology and control of Ebola Virus Disease (EVD) in Sierra Leone: analysis of data from the Médecins Sans Frontières (MSF) response, 2014-15 (Doctoral dissertation, London School of Hygiene & Tropical Medicine).
Castells, M. (2009). Communication Power. Oxford, NY: Oxford University Press.
Centers for Disease Control and Prevention. (2018). The Public Health System and the 10 Essential Public Health Services. Accessed December 2019 at https://www.cdc.gov/publichealthgateway/publichealthservices/essentialhealthservices.html
Centers for Disease Control and Prevention (2024). Drug Resistant TB. Accessed April 2024 at https://www.cdc.gov/tb/topic/drtb/default.htm
Centers for Disease Control and Prevention (2019). Cities Readiness Initiative. Accessed Dec 2019 at https://www.cdc.gov/cpr/readiness/mcm/cri.html
Centers for Disease Control and Prevention Foundation. (2019). What is public health? Accessed November 2019 at https://www.cdcfoundation.org/what-public-health
Centers for Disease Control and Prevention. (2024). Epidemiology Glossary. Accessed March 2024 at https://www.cdc.gov/reproductive-health/glossary/?CDC_AAref_Val=https://www.cdc.gov/reproductivehealth/data_stats/glossary.html
Ceylon, R.F. & Ozkan, B. (2020). The economic effects of epidemics: from SARS and MERS to COVID-19. Research Journal in Advanced Humanities, 1(2), 21-29.
Charrel, R. N., de Lamballerie, X., & Raoult, D. (2007). Chikungunya outbreaks-the globalization of vectorborne diseases. New England Journal of Medicine, 356(8), 769.
Choi, W.S., Kang, C. I., Kim, Y., Choi, J. P., Joh, J. S., Shin, H. S., ... & Kim, Y. S. (2016).
Clinical presentation and outcomes of Middle East respiratory syndrome in the Republic of Korea. Infection & chemotherapy, 48(2), 118-126.
Clement, B., and Cassini, J.P. (2016). Disasters and Public Health: Planning and Response, 2nd Ed. Burlington, MA: Elsevier Inc.
Clifford, G.D., Blaya, J.A., Hall-Clifford, R., and Fraser, H. (2008). Medical Information Systems: A Foundation for Healthcare Technologies in Developing Countries. BioMedical Engineering OnLine, 1-8.
Cole, L. A. (2009). The anthrax letters: A bioterrorism expert investigates the attack that shocked America. Skyhorse Publishing.
Comfort, L.K. (2007). Crisis Management in Hindsight: Cognition, Communication, Coordination, and Control. Public Administration Review(Special Issue) 189-197.
Cooper, L., Kang, S. Y., Bisanzio, D., Maxwell, K., Rodriguez-Barraquer, I., Greenhouse, B., ... & Eckhoff, P. (2019). Pareto rules for malaria super-spreaders and super-spreading. Nature communications, 10(1), 1-9.
Cutler, S.J., Fooks, A.R., & Van der Poel, W.H.M. (2010). Public Health Threat of New, Reemerging, and Neglected Zoonoses in the Industrialized World. Emerging Infectious Disease, 16 (1), 1-7. DOI: 1, 0.3201/eid1601.081467
Cyber Security and Infrastructure Security Agency (CISA), U.S. Department of Homeland Security. Critical Infrastructure Sectors: Public Health and Healthcare. Accessed 10 Jan. 2020 at https://www.cisa.gov/healthcare-and-public-health-sector
Daude, E., Mazumdar, S., & Solanki, V. (2017). Widespread fear of dengue transmission but poor practices of dengue prevention: A study in the slums of Delhi, India. PloS one, 12(2), e0171543.
Delamater, P. L., Street, E. J., Leslie, T. F., Yang, Y. T., & Jacobsen, K. H. (2019). Complexity of the basic reproduction number (R0). Emerging infectious diseases, 25(1), 1.
DHS (2005). “An International Perspective on Advancing Technologies and Strategies for Managing Dual- Use Risks: Report of a Workshop,” Department of Homeland Security, Centers for Disease Control and Prevention, Food and Drug Administration, National Institute of Allergy and Infectious Diseases, National Science Foundation
Dollar, D. (2001). Is globalization good for your health? Bulletin of the World Health Organization, 79, 827-833.
Douglas, P.K., Douglas, D.B., Harrigan, D.C., & Douglas, K.M. (2009). Preparing for Pandemic Influenza and its Aftermath: Mental Health Issues Considered. International Journal of Emergency Mental Health, 11(3).
Farmer, P., & Gastineau, N. (2005). Rethinking Health and Human Rights: Time for a Paradigm Shift. In Gruskin, S., Grodin, M.A., Annas, G.J. & Marks, S.P. (Eds.) Perspectives on Health and Human Rights. Routledge, Taylor & Francis Group.
Farmer, P. (2004). The Pathologies of Power. University of California Press.
Fauci, A. S. (2012). Research on highly pathogenic H5N1 influenza virus: the way forward. Microbiology, 3(5), e00359-12.
Federal Emergency Management Agency (FEMA) (2019). DRAFT, W. (2019). National response framework.
Feldmann-Jensen, S. (2014). Conditions for an App Serving Unplanned Urban Communities: Integrating Cell Phones into Health Promotion Messaging. University of Southern California.
Feldmann-Jensen, S. & Kim, A. (2024). The First Pandemic of the 21st Century: The use of PODs in the 2009 Public Health Response. In Feldmann-Jensen, S., Jensen, S.J., & Slick, J. (Eds.) Case Studies in Disaster Response. Elsevier. https://doi.org/10.1016/B978-0-12-809526-3.00012-9
Fenton, E., Chillag, K., & Michael, N.L. (2015). Ethics preparedness for public health emergencies: recommendations from the presidential bioethics commission. The American Journal of Bioethics, 15(7), 77-79.
Fidler D.P. & Gostin, L.O. (2008). Biosecurity in the Global Age: Biological Weapons, Public Health, and the Rule of Law. Stanford University Press.
Fischer, M.C. (2009). Novel H1N1 Pandemic: When Pigs Fly. The Pediatric Infectious Disease Journal 28(10), 911-914,
Food and Drug Administration (FDA). What Are Medical Countermeasures? FDA Medical Countermeasures Initiative (MCMi). Accessed 10-Jan-2020 at https://www.fda.gov/emergency-preparedness-and-response/about-mcmi/what-are-medical-countermeasures
Fouchier, R.A., García-Sastre, A., Kawaoka, Y., Barclay, W.S., Bouvier, N.M., Brown, I.H., ... & Cox, N.J. (2013). Transmission studies resume for avian flu. Science, 339(6119), 520-521.
Fouchier, R.A., Herfst, S., & Osterhaus, A.D. (2012). Restricted data on influenza H5N1 virus transmission. Science, 335(6069), 662-663.
Garrett, L. (2005). The Next Pandemic? Foreign Affairs, 84(4), 3-23.
Gates, B. (2022). How to Prevent the Next Pandemic. Alfred A. Knopf; New York, NY.Fisc
Goodwin, R., Haque, S., Neto, F., and Myers, L.B. (2009). Initial Psychological Responses to Influenza A, H1N1 (“Swine flu”). BMC Infectious Diseases, 9, 166. DOI:10.1186/1471-2334-9-166
Gostin, L. O., & Nuzzo, J. B. (2021). Twenty years after the anthrax terrorist attacks of 2001: Lessons learned and unlearned for the COVID-19 response. JAMA, 326(20), 2009-2010.
Gould, D. W., Walker, D., & Yoon, P. W. (2017). The evolution of BioSense: lessons learned and future directions. Public Health Reports, 132(1_suppl), 7S-11S.
Graham, B., Talent, J., Larsen, R., & Kidder, L. (2011). Bio-Response Report Card. WMD Center. DIANE Publishing.
Gray, A., & Sharara, F. (2022). Global and Regional Sepsis and Mortality in 2019: A Systematic Analysis. The Lancet-Global Health. DOI: https://doi.org/10.106/S2214-109X(22)00131-0
Griffiths, K., Moise, K., Piarroux, M., Gaudart, J. …Rebaudet, S. (2021). Delineating and Analyzing Locality-level Determinants of Cholera, Haiti. Emerging Infectious Diseases 27(1), 170-181.
Groza, O. (2023). Ebola Ten Years On: Strengthening Global Health Systems and Bridging the Gap. Yale Institute for Global Health.
Guilleman, J. (2005). Biological Weapons: From the Invention of State-sponsored Programs to Contemporary Bioterrorism. Columbia University Press.
Ha, K.M. (2016). A lesson learned from the MERS outbreak in South Korea in 2015. Journal of Hospital Infection, 92(3), 232-234.
Hampton, T. (2013). Report reveals scope of US antibiotic resistance threat. Jama, 310(16), 1661-1663.
Hassan, R., Jabeen, K., Ali, A., Rafiq, Y., Laiq, R., Malik, B., Tanveer, M., Groenheit, R., Ghebremichael, S., Hoffner, S., & Hasan, Z. (2010). Extremely Drug Resistant Tuberculosis, Pakistan. Emerging Infectious Disease 16(9).
Health and Human Services, U.S. Department of (HHS) (2019a). Emergency Preparedness and Response. Accessed January 2020 at https://www.hhs.gov/programs/emergency-preparedness/index.html
Health and Human Services, U.S. Department of (HHS) (2019b). Chemical, Biological, Radiological, and Nuclear (CBRN) Threat Programs. Accessed January 2020 at https://www.medicalcountermeasures.gov/BARDA/CBRN.aspx
Health and Human Services, U.S. Department of (HHS), Assistant Secretary for Preparedness and Response (ASPR) (2019a). Strategic National Stockpile. Accessed December 2019 at https://www.phe.gov/about/sns/Pages/default.aspx
Health and Human Services, Office of Assistant Secretary of Preparedness and Response (ASPR) (2019b). National Disaster Medical System. Accessed at https://www.phe.gov/Preparedness/responders/ndms/Pages/default.aspx
Henderson, D.A. (2009). Smallpox: The Death of a Disease. Promethius Books.
Heyman, D.L. (2003). The Evolving Infectious Disease Threat: Implications for National and Global Security. Journal of Human Development 4(2).
Heyman, D.L., Ed. (2008). Control of Communicable Diseases Manual. Washington D.C.: American Public Health Association.
Heymann, D. L. (2017). Global Health Security. The Global Outbreak Alert and Response Network.
Hyde, J. K., & Shortell, S. M. (2012). The structure and organization of local and state public health agencies in the US: a systematic review. American journal of preventive medicine, 42(5), S29-S41.
Institutes of Medicine (US). (1998). The Future of Public Health, Appendix A. In Committee for the Study of the Future of Public Health, Summary of the Public Health System in the United States. National Academies Press. Available from: https://www.ncbi.nlm.nih.gov/books/NBK218212/
Institutes of Medicine. (2003). Preparing for the Psychological Consequences of Terrorism: A Public Health Strategy. Committee on Responding to the Psychological Consequences of Terrorism; The National Academies Press.
Institutes of Medicine. (2004). Learning from SARS: Preparing for the Next Disease Outbreak Workshop Summary. The National Academies Press.
Institutes of Medicine. (2006). The Impact of Globalization on Infectious Disease Emergence and Control. Forum on Microbial Threats. The National Academies Press.
Institutes of Medicine. (2009). The Domestic and International Impacts of the 2009 H1N1 Influenza A Pandemic: Global Challenges, Global Solutions. National Academies Press: Workshop Summary. http://www.nap.edu/catalog/12799.html
Institutes of Medicine; Stroud C., Viswanathan K., Powell T., Bass R.R. (Eds.). (2011). Prepositioning Antibiotics for Anthrax. National Academies Press.
Jack, A. (2015). Why the panic? South Korea’s MERS response questioned. British Medical Journal, 350, h3403.
Jamison, P., Bogich, T., Elwood, S., Finoff, D.C., & Daszak, P. (2014). Economic Optimization of a Global Strategy to Address the Pandemic Threat. In Proceedings of the NationalAcademy of Sciences, 111(52), 18519–18523.
Joo, H., Maskery, B.A., Berro, A. D., Rotz, L. D., Lee, Y. K., & Brown, C. M. (2019). , Economic impact of the 2015 MERS outbreak on the Republic of Korea's tourism-related industries. Health security, 17(2), 100-108.
Jiang, F., & Doudna, J. A. (2017). CRISPR–Cas9 structures and mechanisms. Annual review of biophysics, 46, 505-529.
Katzelnick, L.C., Narvaez, C., Arguello, S., Lopez Mercado, B., Collado, D., Ampie, O., ... & Harris, E. (2020). Zika virus infection enhances future risk of severe dengue disease. Science, 369(6507), 1123-1128.
Keita, M., Boland, S. T., Okeibunor, J., Chamla, D., Gueye, A. S., & Moeti, M. (2024). 10 years after the 2014–16 Ebola epidemic in west Africa: advances and challenges in African epidemic preparedness. The Lancet.
Kendall,C., Huddelson, P., Leontsini, E., Winch, P., Lloyd, L., and Cruz, F. . (1991). Urbanization, Dengue and Health Transition: Anthropological Contributions to International Health. Medical Anthropology Quarterly Vol. 5 No. 3, 257-268.
Kilerby, M.E., Biggs, H. M., Midgley, C. M., Gerber, S. I., & Watson, J. T. (2020). Middle East respiratory syndrome coronavirus transmission. Emerging infectious diseases, 26(2), 191.
Klobucista, C., & Renwick, D. (2022). Why Hasn’t the World Eradicated Polio? Council on Foreign Relations. Accessed at https://www.cfr.org/backgrounder/why-hasnt-world-eradicated-polio
Kluger, J. (2015). Who’s Afraid of a Little Vaccine? TIME The Science of Epidemics special edition, 72-77.
Knobler, S. L., Mack, A., Mahmoud, A., & Lemon, S. M. (2005). Summary and assessment. In The Threat of Pandemic Influenza: Are We Ready? Workshop Summary. National Academies Press; Washington D.C.
Lancee, W. J., Maunder, R. G., & Goldbloom, D. S. (2008). Prevalence of psychiatric disorders among Toronto hospital workers one to two years after the SARS outbreak. Psychiatric Services, 59(1), 91-95.
Lee, A. (2003). Will the SARS epidemic recur? Host and environment are key factors. Journal of Epidemiology & Community Health, 57(10), 770-771. http://dx.doi.org/10.1136/jech.57.10.770
Lee, S.E., Greene, S.A., Burns, C.C., Tallis, G., Wassilak, S.G.F., & Bolu, O. (2023). Progress Toward Poliomeylitis Eradication- Worldwide, January 2021-March 2023. MMWR. DOI: 10.15585/mmwr.mm7219a3
Lewis, D. (2021). Superspreading drives the COVID pandemic--and could help to tame it. Nature, 590(7847), 544-547.
Liu, E.C. (2009). Would an Influenza Pandemic Qualify as a Major Disaster under the Stafford Act? Congressional Research Service; Washington D.C.
Long, C.M. & Marzi, A. (2021). Biodefence research two decades on: worth the investment? The Lancet Infectious Diseases, 21(8), e222-e233.
Maciosek, M. V., Coffield, A. B., Flottemesch, T. J., Edwards, N. M., & Solberg, L. I. (2010). Greater use of preventive services in US health care could save lives at little or no cost. Health Affairs, 29(9), 1656-1660.
MacNeil, A., Famon, E.C., Wamala, J., Okware, S., Cannon, D.L., Reed, Z., Towner, J.S., Tappero, J.W., Lutwama, J., Downing, R., Nichol, S.T., Ksiazek, T.G., and Rollin, P.E.. (2010). Case Fatality Proportion of Deaths for Infection with Ebola Hemorrhagic Fever, Uganda. Emerging Infectious Disease. DOI: 10.3201/eid1612.100627
McEntire, D.M. & SFHEA. (2015). The Dallas Ebola Incident as an Indicator of the Bioterrorism Threat: An Assessment of Response with Implications for Security and Response. Utah Valley University Journal of National Security 3(2), 5-18.
McGill, N. (2015). Public health looks to lessons from past in MERS outbreak: Experience with SARS offers insights. The Nation’s Health 45(6) 1-12.
McKibbin, W., & Fernando, R. (2023). The global economic impacts of the COVID-19 pandemic. Economic Modelling, 129, 106551.
McLeman, R. (2019). International migration and climate adaptation in an era of hardening borders. Nature Climate Change, 9(12), 911-918.
Memish, Z. A., Assiri, A., Turkestani, A., Yezli, S., al Masri, M., Charrel, R., ... & Gautret, P. (2015). Mass gathering and globalization of respiratory pathogens during the 2013 Hajj. Clinical Microbiology and Infection, 21(6), 571-e1.
Meselson, M.; Guillemin, J.; Hugh-Jones, M.; Langmuir, A.; Popova, I.; Shelokov, A.; Yampolskaya, O. The Sverdlovsk Anthrax Outbreak of 1979. Science 1994, 266, 1202–120.
Migration Policy and Research Program. (2005). Health and Migration: Bridging the Gap. International Organization for Migration; Geneva.
MMWR, (2010). Cholera Outbreak-Haiti, October, 2010. Morbidity and Mortality Weekly Report. U.S. Department of Health and Human Services.
Montgomery, M. (2010). The Demographics of Urbanization in Poor Countries. In In Vlahov, D.B., Boufford, J.I., Pearson, C., & Norris, L.(Eds.), Urban Health: Global Perspectives. Jossey-Bass.
Morens, M., Folkers, G.K., and Fauci, A.S. (2004). The Challenge of Emerging and Re-emerging Infectious Diseases. Nature, 430, 242-249.
Nabel, G. J., & Fauci, A. S. (2010). Induction of unnatural immunity: prospects for a broadly protective universal influenza vaccine. Nature medicine, 16(12), 1389-139.
Naggie, S., Boulware, D. R., Lindsell, C. J., Stewart, T. G., Slandzicki, A. J., Lim, S. C., ... & Patel, Y. (2023). Effect of higher-dose ivermectin for 6 days vs placebo on time to sustained recovery in outpatients with COVID-19: a randomized clinical trial. JAMA, 329(11), 888-897.
National Center for Biotechnology Information (NCBI) (2017). Polymerase Chain Reaction (PCR). National Institutes of Health; Washington, D.C.
National Research Council. (2004). Policy, Global Affairs, Security, Cooperation, Committee on Research Standards & Practices to Prevent the Destructive Application of Biotechnology. Biotechnology research in an age of terrorism. National Academies Press.
National Research Council. (2005). Board on Global Health, Global Affairs, Security, Cooperation, Committee on Advances in Technology, & the Prevention of Their Application to Next Generation Biowarfare Threats. An international perspective on advancing technologies and strategies for managing dual-use risks: report of a workshop. National Academies Press.
National Research Council. (2009). Results of the Survey. In A Survey of Attitudes and Actions on Dual Use Research in the Life Sciences: A Collaborative Effort of the National Research Council and the American Association for the Advancement of Science. National Academies Press (US).
Nuzzo, J. B., & Gronvall, G. K. (2011). Global Health Security: Closing the gaps in responding to infectious disease emergencies. Glob Health Gov IV.
Nye, J.S., and Donahue, J.D. . (2000). Governance in a Globalizing World. Washington D.C.: Brookings Institution Press.
Olaitan, A. O., Morand, S., & Rolain, J. M. (2016). Emergence of colistin-resistant bacteria in humans without colistin usage: a new worry and cause for vigilance.
Osterholm, M.T. (2005). Preparing for the Next Pandemic. Foreign Affairs, 84(4), 24-37.
Osterholm, M.T. (2007). Unprepared for a Pandemic. Foreign Affairs, 86 (2), 47-57.
Osterholm, M.T. and Olshaker, M. (2020). Chronicle of a Pandemic Foretold: Learning from the COVID-19 Failure before the Next Outbreak Arrives. Foreign Affairs, 99(4), 10-24.
O’Sullivan, T.M. (2017). “Mitigating Extreme Infectious Disease Disaster Risk”. Chapter in Bier, V. (Ed.) Risk in Extreme Environments: Preparing, Avoiding, Mitigating, and Managing (pp. 162-173). Routledge.
O'Sullivan, T.M., & Ramsay, J.D. (2021). Public health security (pp.208-230). In Ramsay, J.D., Cozine, K., & Comiskey, J. (Eds.). Theoretical foundations of homeland security: strategies, operations, and structures. Routledge; New York, NY.
O’Sullivan, T.M. (2023). Biological security and bioterrorism: Infectious disease-related risk, theory, practice, and education. Journal of Security, Intelligence & Resilience Education (JSIRE), 17 (10)
Parida, S.K., Axelsson-Robertson, R., Rao, M.V., Singh, N., Master, Il, Lutckii, A., …& Maeurer, M. (2015). Totally drug-resistant tuberculosis and adjunct therapies. Journal of Internal Medicine, 277(4), 388-405.
Park, H.Y., Lee, E.J., Ryu, Y.W., Kim, Y., Kim, H., Lee, H., & Yi, S.J. (2015). Epidemiological investigation of MERS-CoV spread in a single hospital in South Korea, Eurosurveillance, 20(25), 21169.
Parums, D.V. (2024). Climate Change and the Spread of Vector Born Diseases, Including Dengue, Malaria, Lyme Disease, and West Nile Virus Infection. Medical Science Monitor 30. DOI: 10.12659/MSM.943546
Patrick, S. (2020). When the System Fails: COVID-19 and the Costs of Global Dysfunction. Foreign Affairs, 99(4), 40-50.
Patrick W.C. (1996). Biological Terrorism and Aerosol Dissemination. Politics and the LifeSciences.;15(2):208-210. Cambridge. doi:10.1017/S0730938400022826
Paules, C.I., & Fauci, A.S. (2019). Influenza vaccines: good, but we can do better. The Journalof infectious diseases, 219(Supplement 1), S1-S4.
Pita, R., & Gunaratna, R. (2010). Anthrax as a biological weapon: from World War I to the Amerithrax investigation. International journal of intelligence and counterintelligence, 23(1), 61-103.
Pradelle, A., Mainbourg, S., Provencher, S., Massy, E., Grenet, G., & Jean-Christophe, L.E.G.A. (2024). Deaths induced by compassionate use of hydroxychloroquine during the first COVID-19 wave: an estimate. Biomedicine & Pharmacotherapy, 171, 116055.
Price-Smith, A.T. (2009). Contagion and Chaos: Disease, Ecology, and National Security in the Era of Globalization. MIT Press.
Quinn, T., & Bartlett, J. (2010). Global Infectious Diseases and Urbanization. In Vlahov, D.B., Boufford, J.I., Pearson, C., & Norris, L.(Eds.), Urban Health: Global Perspectives (pp. 106-123). Jossey-Bass.
Rai, A., & Khan, T. (2018). Overview of Drug Resistant Mycobacterium tuberculosis. International Journal of Life Science and Scientific Research, 4(3), 1795-1800.
Ravi, S., & Adalja, A.A. (2017). Strengthening the US medical countermeasure enterprise for biological threats. Health security, 15(1), 12-14.
Raviola, G., Eustache, E., Oswald, C., and Belkin, G.S. (2012). Mental Health Response in Haiti in the Aftermath of the 2010 Earthquake: A Case Study for Building Longterm Solutions. Harvard Review Psychiatry 20, 68-77.
Rigaud, K.K., Jones, B., Bergmann, J., Clement, V., Ober, K., Schewe, J., ... & Midgley, A. (2018). Groundswell: Preparing for Internal Climate Migration. World Bank.
Robert Wood Johnson Foundation. (2009). Ready or Not? Protecting the Public’s Health from Diseases, Disasters, and Bioterrorism. Issue Report. Trust for America’s Health.
Rose, D.A., Murthy, S., Brooks, J., & Bryant, J. (2017), The Evolution of Public Health Emergincy Management as a Field of Practice. American Journal of Public Health 107(S2), S126-133.
Roser, M. (2024). The Global Fight Against Polio – How Far Have We Come? Our World inData. Accessed at https://ourworldindata.org/global-fight-polio#article-citation
Rotz, L.D., Khan, A.S., Lillibridge, S.R., Ostroff, S.M., & Hughes, J.M. (2002). Public health assessment of potential biological terrorism agents. Emerging Infectious Disease, 8, 225-230.
Ruiz-Aravena M, McKee C, Gamble A, Lunn T, Morris A, Snedden CE, Yinda CK, Port JR, Buchholz DW, Yeo YY, Faust C, Jax E, Dee L, Jones DN, Kessler MK, Falvo C, Crowley D, Bharti N, Brook CE, Aguilar HC, Peel AJ, Restif O, Schountz T, Parrish CR, Gurley ES, Lloyd-Smith JO, Hudson PJ, Munster VJ, Plowright RK (2022). Ecology, evolution and spillover of coronaviruses from bats. Nat Rev Microbiol. 2022 May;20(5):299-314. Erratum in: Nat Rev Microbiol. 2022 May;20(5):315. doi: 10.1038/s41579-022-00691-3.
Ryan, E.T. (2011). Haiti in the Context of the Current Global Cholera Pandemic. Emerging Infectious Disease, 17(11), 2175-2176.
Saker, L., Lee, K., Cannito, B., Gilmore, A., Campbel, D. (2004). Globalization and Infectious Disease: A Review of the Linkages. World Health Organization, The Special Program for Research and Training in Tropical Diseases (TDR), & World Bank.
Samet, J.M., & Woodward, A. (2018). National Government Denial of Climate Change and State and Local Public Health Action in a Federalist System. American Journal of Public Health 108 (Suppl 2). doi 10.2105/AJPH.2018.304395
Schmitt, K., & Zacchia, N. A. (2012). Total decontamination cost of the anthrax letter attacks. Biosecurity and bioterrorism: biodefense strategy, practice, and science, 10(1), 98-107.
Shah, S. (2016). Pandemic. Picador.
Shelton, S. R., Connor, K., Uscher-Pines, L., Pillemer, F. M., Mullikin, J. M., & Kellermann, A. L. (2012). Bioterrorism and biological threats dominate federal health security research; other priorities get scant attention. Health Affairs, 31(12), 2755-2763.
Silver, C. B., Leon, E. L., Zaino, S. A., & Mandel, E. D. (2015). Ebola: Lessons from the latest pandemic. Clinician Reviews, 25(9), 34-40.
Starshinova, A., Dovgalyk, I., Beltukov, M., Zinchenko, Y., Glushkova, A., Starshinova, A.Y., Doktorova, N., and Kudlay, D. (2022). Tuberculosis in the Russian Federation: Dynamics of the Epidemic Indicators before and after COVID-19 Pandemic. Life (Basel), 10, 1468. doi: 10.3390/life12101468
Stern, J., & Shouten, R. (2016). Lessons from the Anthrax Letters. In Bunn, M., & Sagan, S. D. (Eds.). (2017). Insider threats. Cornell University Press.
Suk, J.E., Zmorzynska, A., Hunger, I., Biederbick, W., Sasse, J., Maidhof, H., & Semenza, J.C. (2011). Dual-use Research and Technological Diffusion: Reconsidering the Bioterrorism Threat Spectrum. PLoS Pathogens, 7(1).
Taubenberger, J.K., & Morens, D.M. (2006). Influenza revisited. Emerging infectious diseases, 12(1), 1.
The COVID Crisis Group. (2023). Lessons from the COVID War: An Investigative Report. Hachette Book Group Inc.
Trainor, J., Aguirre, B.E., Barnshaw, J. (2008). Social Scientific Insights on Preparedness for Public Health Emergencies. University of Delaware Disaster Research Center.
Tucker, J.B. (2004). Biological threat assessment: is the cure worse than the disease? ArmsControl Today, 34(8), 13-19.
Tucker, J.B., & Zilinskas, R.A. (2006). The promise and perils of synthetic biology. The New Atlantis, (12), 25-45.
United Nations HABITAT. (2007). State of the World's Cities Report: Urban penalty the poor die young. UN-HABITAT.
United Nations High Commission for Refugees (UNHCR). (2019). Climate Change and Disaster Displacement. Accessed December 2019 at https://www.unhcr.org/climate-change-and-disasters.html
United States of America Whitehouse. (2022). National Biodefense Strategy and Implementation Plan: for Countering Biological Threats, Enhancing Pandemic Preparedness, and Achieving Global Health Security. Whitehouse; Washington D.C,
Van Bortel, T., Basnayake, A., Wurie, F., Jambai, M., Koroma, A. S., Muana, A. T., ... & Nellums, L. B. (2016). Psychosocial effects of an Ebola outbreak at individual, community and international levels. Bulletin of the World Health Organization, 94(3), 210.
Van Seventer, J.M., & Hochberg, N.S. (2017). Principles of Infectious Diseases: Transmission, Diagnosis, Prevention, and Control. International Encyclopedia of Public Health, 2nd Ed., 6. http://dx.doi.org/10.1016/B978-0-12-803678-5.00516-6
Venkatesan, P. (2022). Global Polio Eradication Set Back by COVID-19 Pandemic. Lancet 3(3). DOI: https://doi.org/10.1016/S2666-5247(22)000042-8
Walker, B. (1989). The future of public health: the Institute of Medicine's 1988 report. Journal ofpublic health policy, 10(1), 19-31.
Wang, Q., Sun, L., & Jiang, S. (2023). Potential recombination between SARS-CoV-2 and MERS-CoV: calls for the development of Pan-CoV vaccines. Signal Transduction and Targeted Therapy, 8(1), 122.
Wappes, J. (2016). Highly resistant MCR-1 ‘superbug’ found in U.S. for first time. CIDRAP http://www.cidrap.umn.edu/news-perspective/2016/05/highly-resistant-mcr-1-superbug-found-us-first-time
Wein, L.M., & Liu, Y. (2005). Analyzing a bioterror attack on the food supply: the case of botulinum toxin in milk. Proceedings of the National Academy of Sciences, 102(28), 9984-9989.
Wong, G., Liu, W., Liu, Y., Zhou, B., Bi, Y., & Gao, G. F. (2015). MERS, SARS, and Ebola: the role of super-spreaders in infectious disease. Cell host & microbe, 18(4), 398-401.
Wongsrichanalai, C., Varma, J.K., Juiano, J.J., Kimerling, M.E., & MacArthur, J.R. (2010). Extensive Drug Resistance in Malaria and Tuberculosis. Emerging Infectious Disease 16 (7).
World Health Organization (WHO). (2005). WHO Global Influenza Preparedness Plan: therole of WHO and recommendations for national measures before and during pandemics.
World Health Organization (WHO). (2010). Public Health Risk Assessments and Interventions:Earthquake, Haiti.
World Health Organization (WHO). (2015). Intensified public health measures help control MERS-CoV outbreak in the Republic of Korea. WHO. Accessed at http://www.wpro.who.int/mediacentre/releases/2015/20150728/en/
World Health Organization (WHO). (2017). Vector-borne diseases. Accessed at https://www.who.int/news-room/fact-sheets/detail/vector-borne-diseases
World Health Organization (WHO). (2023). Global Tuberculosis Report 2023. https://www.who.int/teams/global-tuberculosis-programme/tb-reports
World Health Organization (WHO). (2023). Tuberculosis Key Facts. Accessed April 2024 at https://www.who.int/news-room/fact-sheets/detail/tuberculosis
World Health Organization (WHO). (2024). Social Determinants of Health. WHO. Accessed March 2024 at https://www.who.int/health-topics/social-determinants-of-health#tab=tab_1
World Health Organization (WHO). (2024). Global Health Estimates: Leading Causes of DALYs. WHO. Accessed March 2024 at https://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates/global-health-estimates-leading-causes-of-dalys
World Health Organization (WHO). (2024). Leading Causes of Death Globally. WHO.Accessed March 2024 at https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death
You, W., &Donnelly, F. (2024). Nursing workforce plays a significant role in reducing COVID‐19 deaths worldwide: A cross‐sectional analysis of data from 178 countries. Nursing & Health Sciences, 26(1), e13099.
Xiong, J., Lipsitz, O., Nasri, F., Lui, L. M., Gill, H., Phan, L., ... & McIntyre, R. S. (2020). Impact of COVID-19 pandemic on mental health in the general population: A systematic review. Journal of affective disorders, 277, 55-64.
Zeng, Z., Zhan, J., Chen, L., Chen, H., & Cheng, S. (2021). Global, regional, and national dengue burden from 1990 to 2017: A systematic analysis based on the global burden of disease study 2017. eClinicalMedicine, 32.
Zumla, A., Peiris, M., Memish, Z. A., & Perlman, S. (2024). Anticipating a MERS-like coronavirus as a potential pandemic threat. The Lancet, 403(10438), 1729-1731.
- It went on to note that American policy should be aimed at developing a new plan for bioweapons proliferation and bioterrorism prevention, emphasizing renewal of the neglected and insufficient 1972 Biological Weapons Convention (BWC). The Commission also urged renewed efforts to evaluate domestic sources of dangerous disease agents, and improved oversight for research labs from which germs might be stolen or accidentally released. (WMD Commission 2008). ↵

