9 The Vaginal Microbiome
The Vaginal Microbiome
The vaginal microbiome is a reproductive organ-specific niche that harbors a unique collection of microorganisms that are important in a variety of aspects to human health. This microbial community has significance in maintaining vaginal homeostasis, protecting against urogenital infections, host immunity, and reproductive capacities. In recent years, next generation sequencing (NGS) techniques have been employed to classify the vaginal microbiome into community state types (CSTs), or vaginotypes, based upon composition, which can enhance epidemiological studies to make better associations between the microbiome and host-vaginal health (France et al., 2020, Mancabelli et al., 2021).
Composition and Role
The vaginal microbiome is composed of over 200 different bacterial species, though it is primarily dominated by members from Lactobacillus genus (Ma et al., 2012, Auriemma et al., 2021). The Lactobacilli protect the vagina from potential pathogen invasion through their fermentation of vaginal epithelial cell-produced glycogen into lactic acid, which lowers vaginal pH, as well as their production of various antimicrobial compounds, and resource/space competitive inhibition (Boskey et al., 2001, Amabebe and Anumba, 2018, Chee et al., 2020, Jang et al., 2019). Though, the bacteriome is usually the emphasized feature of the vaginal microbiome, it also harbors protists, fungi, archaea and viruses, with each occupying their own niche in the normal healthy network (Belay et al., 1990, Bradford and Ravel, 2017, Happel et al., 2020, Chacra and Fenollar, 2021). The composition isn’t always fixed however, as factors like the menstrual cycle, hormone fluctuation, sexual partners, hygiene, genetics, age, the environment, drug use, and other aspects of lifestyle can affect the microbial makeup (Aagaard et al., 2012, Fettweis et al., 2014, Hyman et al., 2014, Zapata and Quagliarello, 2015, Martin and Marrazzo, 2016, Diop et al., 2019, Chacra and Fenollar, 2021).
As mentioned earlier, using 16S rRNA sequencing, the vaginal microbiome has been categorized into CSTs and allowed for deeper analysis, where comparisons of the consortia within these classifications can be used to make associations with host and vaginal health. There are 5 main CSTs each predominated by a particular species of Lactobacilli, except for group IV, which has been further dissected into additional subgroups: CST I—L. crispatus dominated, CST II—L. gasseri dominated, CST III—L. iners dominated, and CST V—L. jensenii dominated (Ravel et al., 2011, France et al., 2020, Chacra and Fenollar, 2021, Mancabelli et al., 2021). CST IV is not dominated by any particular species, and contains a mixture of both strict and facultative anaerobes including Gardnerella, Atopobium, Lactobacillus, Bifidobacterium, etc., where certain controversial subgroups (e.g. CST IV-A, CST IV-B, CST IV-C, CST IV-D, CST IV-G, etc.) have various combinations depending on the study (Gajer et al., 2012, Albert et al., 2015, France et al., 2020, Mancabelli et al., 2021). Each of the five groups has correlations with vaginal pH, microbial colonization and biodiversity, as well as host characteristics such as pregnancy, ethnicity, and age, though it is not always absolute (France et al., 2020, Mancabelli et al., 2021). With reproducible organization of the vaginal microbiome, connections can be made with medical conditions, which could benefit treatment and diagnosis of vaginal diseases and associated affairs.
Dysbiosis and Disease
As with other microbiomes associated with the human body, disturbance of the resident vaginal microbiome can result in complications of health. Many diseases of the urogenital tract such as bacterial vaginosis (BV), urinary tract infections (UTIs), yeast infections, and several sexually transmitted infections (STIs) are caused by pathogenic microbes associated with dysbiosis (Taha et al., 1998, Donders et al., 2000, Wiesenfeld et al., 2003; Lai et al., 2009, De Seta et al., 2019, Mancabelli et al., 2021). Normally, the local flora maintains homeostasis, however, changes in composition provide opportunistic pathogens a window to proliferate and invade.
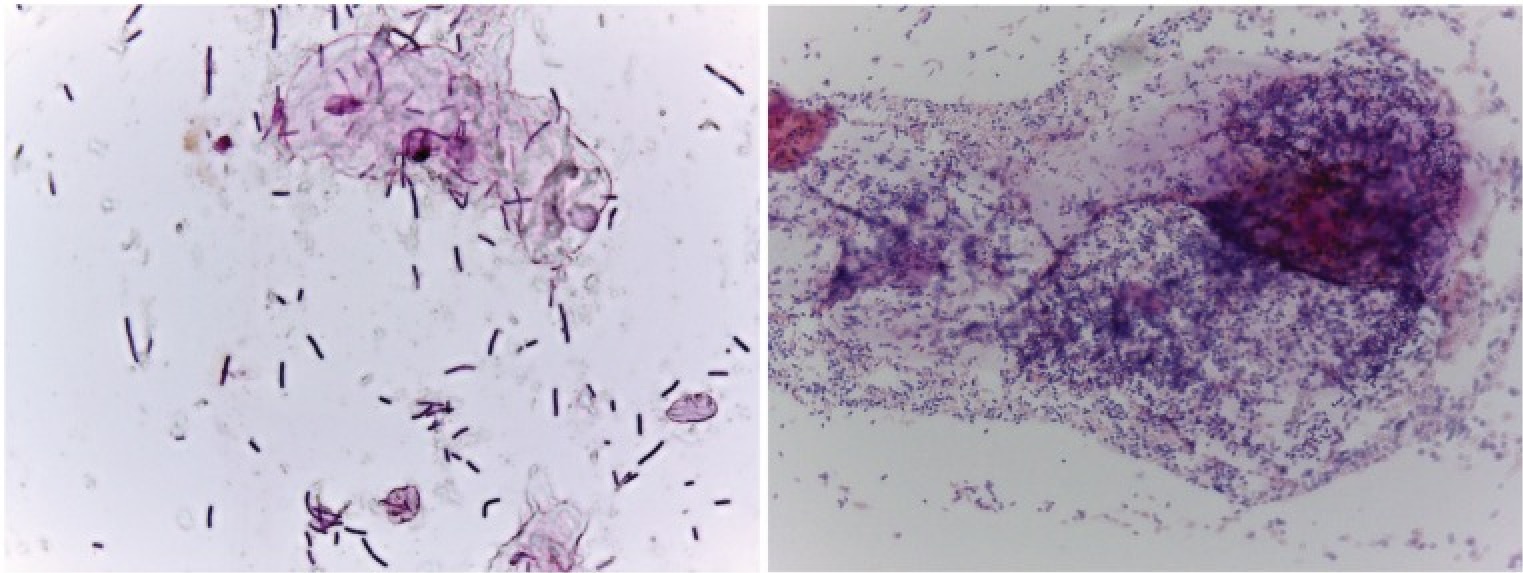
Bacterial vaginosis, associated with CST IV, stems from a perturbance of the vaginal flora, specifically a decrease in Lactobacilli and an increase in other microbes like Gardnerlla vaginalis, Atopboium vaginae, Ureaplasma urealyticum, and others that are only usually found in low numbers (Gajer et al., 2012, Margolis and Fredricks, 2015, Onderdonk et al., 2016, Zozaya et al., 2016). This condition can be asymptomatic in up to half the women with BV, and in the others can be diagnosed by observed changes in vaginal discharge or by the Nugent scoring system which utilizes a Gram stain (Figure 1) (Nugent et al., 1991, Amsel et al., 1983, Schwebke, 2000). While not exactly considered a sexually transmitted disease, bacterial vaginosis is commonly associated with certain sexual practices, though other factors such as hygiene, nutrition, intrauterine devices, hormonal changes, and certain comorbidities can contribute to susceptibility (Avonts et al, 1990, Calzolari et al., 2000, Neggers et al., 2007, Verstraelen et al., 2010, Zabor et al, 2010, Margolis and Fredricks, 2015). Women with this disease also have a higher risk to contract sexually transmitted infections, and it can be linked to reproductive complications and poor infant health (Wiesenfeld et al., 2003, Prince et al., 2015, Chacra and Fenollar, 2021). Typically, antibiotics targeting anaerobic bacteria are administered to treat BV, however recurrence is common, likely due to the antimicrobial-resistant nature of biofilms formed by the pathogens and/or regular exposure to external reservoirs (Swidinski et al., 2008, Oduyebo et al., 2009, Marrazzo et al., 2012, Bradshaw and Sobel, 2016). Probiotics, specifically containing L. crispatus, may be the better option, and vaginal microbiota transplantation from a healthy donors is a promising treatment course of action (Hemmerling et al., 2010, Ma et al., 2019).
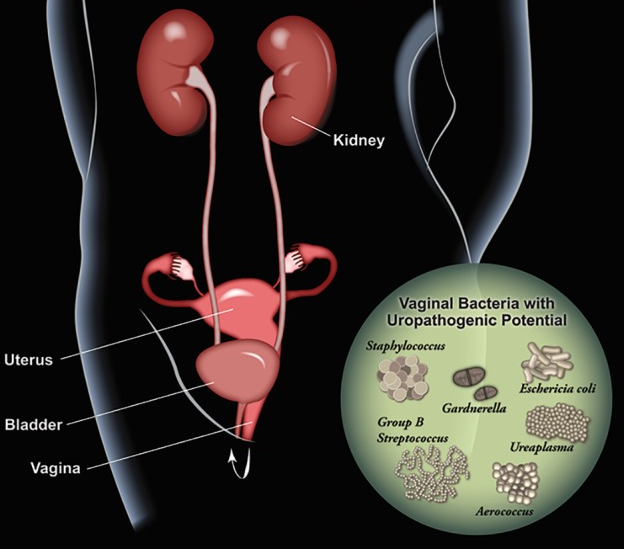
Urinary tract infections are a common problem, especially for females, which are disproportionately affected. This infection is most likely to occur in the urethra or bladder (though in severe cases the kidneys can be affected), which causes pain in the pelvic area and during urination, the frequent urge to urinate, and the presence of blood in urine (Lee and Neild, 2007, Sheerin, 2011, Hooton, 2012). The vaginal microbiome, or more specifically some normal residents, like Escherichia coli, can move to the urogenital tract and cause issues resulting in a UTI, which often occurs via sexual activity (Nicolle et al., 1982, Foxman, 2014, Stapleton, 2016, Lewis and Gilbert, 2020). Other fastidious microorganisms, like those abundant in women experiencing BV, contribute to a higher risk of contracting an infection in the urinary tract as compared with those not suffering from dysbiosis in the vagina (Hillebrand et al., 2002, Sumati and Saritha, 2009). In these cases, the vaginal opening may harbor potential uropathogens (Figure 2) and transient exposure to the urinary tract could prompt colonization or other reactions (e.g. immunomodulation) that results in a UTI (Lewis and Gilbert, 2020). Antibiotics are traditionally used to treat UTIs, however probiotics and estrogen administration could help to restore the Lactobacillus colonization and protect against complicated and recurrent infections (Raz and Stamm, 1993, Eriksen, 1999, Prais et al., 2003, Stapleton et al., 2011, Tan and Chlebicki, 2016, Lewis and Gilbert, 2020).
Yeast infection of the vaginal region caused by Candida species, also known as vulvovaginal candidiasis (VVC), is a common condition with severe symptoms and a high recurrence rate (Oerlemans et al., 2020). Those affected experience vaginal itchiness or soreness, dyspareunia (painful intercourse), abnormal vaginal discharge, redness, swelling, and thinning of the vaginal wall (Chew and Than, 2016, Oerlemans et al., 2020). While it is the second-most common infection of the vagina behind BV, this disease primarily affects premenopausal women with a low vaginal pH value under 4.5 (Kim and Park, 2017, Gupta et al., 2019). The exact cause of VVC isn’t exactly clear, though it is thought it can come as a result of microbiome dysbiosis induced by prolonged antibiotic usage, which allows various Candida species to overgrow and establish an infection (Goldacre et al., 1979, Mitchell, 2004, Peters et al., 2014, van de Wijgert and Verwijs, 2020). Traditionally, antifungal medication has been used to treat VVC, however administration of probiotic vaginal microbes may be more prudent as their mechanisms of pathogen colonization and biofilm formation inhibition are more effective to prevent disease recurrence (Petrova et al., 2016, Tachedjian et al., 2017, Allonsius et al., 2019, Oerlemans et al., 2020, van de Wijgert and Verwijs, 2020).
Individuals with vaginal microbiome dysbiosis characterized by a decrease in abundance of Lactobacilli species (i.e. BV) are at a higher risk of contracting sexually transmitted infections, such as those caused by Neisseria gonorrhoeae, Trichomonas vaginalis, Chlamydia trachomatis, and Mycoplasma genitalium (Martin et al., 1999, Cherpes et al., 2003, Peipert et al., 2008, Brotman et al., 2010, Molenaar et al., 2018, De Seta et al., 2022). Infections caused by these organisms usually result in symptoms similar to other genital infections such as vaginal itching, pain, unusual discharge, rash, etc. which like other conditions (e.g. vaginitis), result in decreased epithelial integrity and can exacerbate pathogen invasion (Miller and Shattock, 2003, Greenbaum et al., 2019). In those individuals with BV and non-inflamed tissue, the increased risk for STIs could be due to the negative effects dysbiosis-related bacteria have on the innate immune system (Murphy and Mitchell, 2016, Liebenberg et al., 2017). Similarly, contraction of sexually transmitted viral infections like human immunodeficiency virus (HIV), herpesviruses, and human papillomavirus (HPV) are frequently associated with vaginal dysbiosis, immunomodulation, and disruption of the epithelial barrier (Sewankambo et al., 1997, Borgdorff et al., 2016, Siqueira et al., 2019, Torcia, 2019, De Seta et al., 2022). While there is correlation between vaginal dysbiosis and STIs, the protective mechanisms of the resident vaginal microbiota are unknown and still need to be uncovered (De Seta et al., 2022).
Reproduction, Pregnancy, and Infant health
The vaginal microbiome has further implications in host immunity, fertility, pregnancy, spontaneous preterm birth, and infant health (Anahtar et al., 2015, Fettweis et al., 2019, Gupta et al., 2020, Xu et al., 2020). Indeed, there are a multitude of factors that affect reproduction like age, genetics, hormone levels, fallopian tube blockage, menstrual cycle, and vaginal pH, however only recently has the vaginal microbiome been studied for its association with various fertility factors (Xu et al., 2020, Fan et al., 2022).
Changes in the resident vaginal flora and infections by certain pathogens can cause complications for reproductive health and pregnancy. For example, infection by Group B Streptococcus (GBS) has been associated with a decline in ovarian function, pregnancy loss, preterm delivery, and is the leading cause of bacteremia and meningitis in newborns (Zaleznik et al., 2000, Phares et al., 2008, Kolter and Henneke, 2017, Tazi et al., 2019, Xu et al., 2020). The vaginal microbiome of pregnant women is less rich and diverse than non-pregnant individuals, likely caused by changes in sex hormone levels (Farage et al., 2010, Aagaard et al., 2012). These alterations can cause shifts in the vaginal microbiome that could then result in infection and a risk of preterm or spontaneous labor (Wylie et al., 2018, Fettweis et al., 2019, Feehily et al., 2020, Gupta et al., 2020).
Infants become introduced to the microbial world via their mother, and predominately right after birth where vaginal versus cesarean delivery has a great impact on composition (Chu et al., 2017). However, exposure may happen earlier in utero, as this environment may not be as sterile as once thought as some studies have shown the presence of microbes in the placenta and amniotic fluid (Aagaard et al., 2014, Collado et al., 2016, Kolter and Henneke, 2017). The establishment of a newborn’s microbiome has profound effects on the development of immunity and metabolism as well as the onset of diseases like atopic dermatitis and obesity in later life (Rautava et al., 2012, Collado et al., 2016, Ta et al., 2020).
Conclusion
The vaginal microbiome is a complex community which affects many facets of human health and disease including urogenital, reproductive, immune, and infant. Characterization of the vaginal flora has allowed categorization into community state types which can help to predict and diagnose disease states. Within so, these dynamic changes that occur under various conditions produce a unique fingerprint for the vaginal microbiome which can be analyzed and potentially treated in a specific manner (Ceccarani et al., 2019, Lagenaur et al., 2020, Abou Chacra and Fenollar, 2021). While antibiotic administration has its benefits, novel approaches using targeted application of probiotic microbiomes (e.g. a gel containing specific Lactobacilli species) could help in treating diseases associated with vaginal dysbiosis by restoring those disrupted communities, as well as alleviating certain negative consequences of chemotherapy (Pino et al., 2019, Lagenaur et al., 2020 Oerlemans et al., 2020). More in-depth and continued research of the vaginal microbiome is necessary to illuminate the interactions and connections of it members to human health.
Drag and Drop Quiz
Drag the species of bacteria that dominates each vaginal community state type.
Check your Understanding
- Explain vaginal microbiome CSTs. Why are they important?
- Which CST is associated with BV, and which genus is typically absent?
- How does BV contribute to the development of other conditions like UTIs, VVC, and STIs?
- How does the vaginal microbiome influence reproduction?
- Where does a newborn acquire its microbiome, and what factors affect the composition?
- What alternatives to antimicrobials are promising for the treatment of vaginal microbiome associated diseases?
Media Attributions
- Video 1 – VALENCIA: A nearest centroid classification for vaginal microbial communities by Research Square. Licensed under Creative Commons: By Attribution 3.0 License https://creativecommons.org/licenses/by/3.0/
- Figure 1 – Examples of Gram-stained slides from women with (right, Nugent score = 9) and without (left, Nugent score = 1) BV by Lewis and Gilbert, 2020. Licensed under Creative Commons Attribution 4.0 License https://creativecommons.org/licenses/by/4.0/
- Figure 2 – Schematic illustrating vaginal bacteria with potential to impact the urinary tract by Lewis and Gilbert, 2020. Licensed under Creative Commons Attribution 4.0 License https://creativecommons.org/licenses/by/4.0/
- Video 2 – Beyond bacterial vaginosis: vaginal lactobacilli and HIV risk by Research Square. Licensed under Creative Commons: By Attribution 3.0 License https://creativecommons.org/licenses/by/3.0/
- Video 3 – A temporal model of cervicovaginal microbiota identifies targets to promote reproductive health by Research Square. Licensed under Creative Commons: By Attribution 3.0 License https://creativecommons.org/licenses/by/3.0/
References
- Aagaard, K., Ma, J., Antony, K. M., Ganu, R., Petrosino, J., & Versalovic, J. (2014). The Placenta Harbors a Unique Microbiome. Science Translational Medicine, 6(237), 237ra65-237ra65. https://doi.org/10.1126/scitranslmed.3008599
- Aagaard, K., Riehle, K., Ma, J., Segata, N., Mistretta, T.-A., Coarfa, C., Raza, S., Rosenbaum, S., van den Veyver, I., Milosavljevic, A., Gevers, D., Huttenhower, C., Petrosino, J., & Versalovic, J. (2012). A Metagenomic Approach to Characterization of the Vaginal Microbiome Signature in Pregnancy. PLOS ONE, 7(6), e36466-. https://doi.org/10.1371/journal.pone.0036466
- Abou Chacra, L., & Fenollar, F. (2021). Exploring the global vaginal microbiome and its impact on human health. Microbial Pathogenesis, 160, 105172. https://doi.org/10.1016/j.micpath.2021.105172
- Albert, A. Y. K., Chaban, B., Wagner, E. C., Schellenberg, J. J., Links, M. G., van Schalkwyk, J., Reid, G., Hemmingsen, S. M., Hill, J. E., Money, D., & Group, V. R. (2015). A Study of the Vaginal Microbiome in Healthy Canadian Women Utilizing cpn60-Based Molecular Profiling Reveals Distinct Gardnerella Subgroup Community State Types. PLOS ONE, 10(8), e0135620-. https://doi.org/10.1371/journal.pone.0135620
- Allonsius, C. N., Vandenheuvel, D., Oerlemans, E. F. M., Petrova, M. I., Donders, G. G. G., Cos, P., Delputte, P., & Lebeer, S. (2019). Inhibition of Candida albicans morphogenesis by chitinase from Lactobacillus rhamnosus GG. Scientific Reports, 9(1), 2900. https://doi.org/10.1038/s41598-019-39625-0
- Amabebe, E., & Anumba, D. O. C. (2018). The Vaginal Microenvironment: The Physiologic Role of Lactobacilli. Frontiers in Medicine, 5, 181. https://doi.org/10.3389/fmed.2018.00181
- Amsel, R., Totten, P. A., Spiegel, C. A., Chen, K. C. S., Eschenbach, D., & Holmes, K. K. (1983). Nonspecific vaginitis: Diagnostic criteria and microbial and epidemiologic associations. The American Journal of Medicine, 74(1), 14–22. https://doi.org/10.1016/0002-9343(83)91112-9
- Anahtar, M. N., Byrne, E. H., Doherty, K. E., Bowman, B. A., Yamamoto, H. S., Soumillon, M., Padavattan, N., Ismail, N., Moodley, A., Sabatini, M. E., Ghebremichael, M. S., Nusbaum, C., Huttenhower, C., Virgin, H. W., Ndung’u, T., Dong, K. L., Walker, B. D., Fichorova, R. N., & Kwon, D. S. (2015). Cervicovaginal Bacteria Are a Major Modulator of Host Inflammatory Responses in the Female Genital Tract. Immunity, 42(5), 965–976. https://doi.org/10.1016/j.immuni.2015.04.019
- Auriemma, R. S., Scairati, R., del Vecchio, G., Liccardi, A., Verde, N., Pirchio, R., Pivonello, R., Ercolini, D., & Colao, A. (2021). The Vaginal Microbiome: A Long Urogenital Colonization Throughout Woman Life. Frontiers in Cellular and Infection Microbiology, 11, 613. https://www.frontiersin.org/article/10.3389/fcimb.2021.686167
- AVONTS, D., SERCU, M., HEYERICK, P., VANDERMEEREN, I., MEHEUS, A., & PIOT, P. (1990). Incidence of Uncomplicated Genital Infections in Women Using Oral Contraception or an Intrauterine Device: A Prospective Study. Sexually Transmitted Diseases, 17(1), 23–29. http://www.jstor.org/stable/44971143
- Belay, N., Mukhopadhyay, B., E, C. de M., Galask, R., & Daniels, L. (1990). Methanogenic bacteria in human vaginal samples. Journal of Clinical Microbiology, 28(7), 1666–1668. https://doi.org/10.1128/jcm.28.7.1666-1668.1990
- Boskey, E. R., Cone, R. A., Whaley, K. J., & Moench, T. R. (2001). Origins of vaginal acidity: high d/l lactate ratio is consistent with bacteria being the primary source. Human Reproduction, 16(9), 1809–1813. https://doi.org/10.1093/humrep/16.9.1809
- Bradford, L. L., & Ravel, J. (2017). The vaginal mycobiome: A contemporary perspective on fungi in women’s health and diseases. Virulence, 8(3), 342–351. https://doi.org/10.1080/21505594.2016.1237332
- Bradshaw, C. S., & Sobel, J. D. (2016). Current Treatment of Bacterial Vaginosis—Limitations and Need for Innovation. The Journal of Infectious Diseases, 214(suppl_1), S14–S20. https://doi.org/10.1093/infdis/jiw159
- Brotman, R. M., Klebanoff, M. A., Nansel, T. R., Yu, K. F., Andrews, W. W., Zhang, J., & Schwebke, J. R. (2010). Bacterial vaginosis assessed by gram stain and diminished colonization resistance to incident gonococcal, chlamydial, and trichomonal genital infection. The Journal of infectious diseases, 202(12), 1907–1915. https://doi.org/10.1086/657320
- Calzolari, E., Masciangelo, R., Milite, V., & Verteramo, R. (2000). Bacterial vaginosis and contraceptive methods. International Journal of Gynecology & Obstetrics, 70(3), 341–346. https://doi.org/https://doi.org/10.1016/S0020-7292(00)00217-4
- Ceccarani, C., Foschi, C., Parolin, C., D’Antuono, A., Gaspari, V., Consolandi, C., Laghi, L., Camboni, T., Vitali, B., Severgnini, M., & Marangoni, A. (2019). Diversity of vaginal microbiome and metabolome during genital infections. Scientific Reports, 9(1), 14095. https://doi.org/10.1038/s41598-019-50410-x
- Chee, W. J. Y., Chew, S. Y., & Than, L. T. L. (2020). Vaginal microbiota and the potential of Lactobacillus derivatives in maintaining vaginal health. Microbial Cell Factories, 19(1), 203. https://doi.org/10.1186/s12934-020-01464-4
- Cherpes, T. L., Meyn, L. A., Krohn, M. A., Lurie, J. G., & Hillier, S. L. (2003). Association between acquisition of herpes simplex virus type 2 in women and bacterial vaginosis. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America, 37(3), 319–325. https://doi.org/10.1086/375819
- Chew, S. Y., & Than, L. T. L. (2016). Vulvovaginal candidosis: contemporary challenges and the future of prophylactic and therapeutic approaches. Mycoses, 59(5), 262–273. https://doi.org/https://doi.org/10.1111/myc.12455
- Chu, D. M., Ma, J., Prince, A. L., Antony, K. M., Seferovic, M. D., & Aagaard, K. M. (2017). Maturation of the infant microbiome community structure and function across multiple body sites and in relation to mode of delivery. Nature Medicine, 23(3), 314–326. https://doi.org/10.1038/nm.4272
- Collado, M. C., Rautava, S., Aakko, J., Isolauri, E., & Salminen, S. (2016). Human gut colonisation may be initiated in utero by distinct microbial communities in the placenta and amniotic fluid. Scientific Reports, 6(1), 23129. https://doi.org/10.1038/srep23129
- de Seta, F., Campisciano, G., Zanotta, N., Ricci, G., & Comar, M. (2019). The Vaginal Community State Types Microbiome-Immune Network as Key Factor for Bacterial Vaginosis and Aerobic Vaginitis. Frontiers in Microbiology, 10, 2451. https://www.frontiersin.org/article/10.3389/fmicb.2019.02451
- de Seta, F., Lonnee-Hoffmann, R., Campisciano, G., Comar, M., Verstraelen, H., Vieira-Baptista, P., Ventolini, G., & Lev-Sagie, A. (2022). The Vaginal Microbiome: III. The Vaginal Microbiome in Various Urogenital Disorders. Journal of Lower Genital Tract Disease, 26(1). https://journals.lww.com/jlgtd/Fulltext/2022/01000/The_Vaginal_Microbiome__III__The_Vaginal.17.aspx
- Diop, K., Dufour, J.-C., Levasseur, A., & Fenollar, F. (2019). Exhaustive repertoire of human vaginal microbiota. Human Microbiome Journal, 11, 100051. https://doi.org/10.1016/j.humic.2018.11.002
- Donders, G. G. G., Bosmans, E., Dekeersmaecker, A., Vereecken, A., van Bulck, B., & Spitz, B. (2000). Pathogenesis of abnormal vaginal bacterial flora. American Journal of Obstetrics and Gynecology, 182(4), 872–878. https://doi.org/10.1016/S0002-9378(00)70338-3
- Eriksen B. (1999). A randomized, open, parallel-group study on the preventive effect of an estradiol-releasing vaginal ring (Estring) on recurrent urinary tract infections in postmenopausal women. American journal of obstetrics and gynecology, 180(5), 1072–1079. https://doi.org/10.1016/s0002-9378(99)70597-1
- Fan, W., Kan, H., Liu, H. Y., Wang, T. L., He, Y. N., Zhang, M., Li, Y. X., Li, Y. J., Meng, W., Li, Q., Hu, A. Q., & Zheng, Y. J. (2022). Association between Human Genetic Variants and the Vaginal Bacteriome of Pregnant Women. MSystems, 6(4), e00158-21. https://doi.org/10.1128/mSystems.00158-21
- Farage, M. A., Miller, K. W., & Sobel, J. D. (2010). Dynamics of the Vaginal Ecosystem—Hormonal Influences. Infectious Diseases: Research and Treatment, 3, IDRT.S3903. https://doi.org/10.4137/IDRT.S3903
- Feehily, C., Crosby, D., Walsh, C. J., Lawton, E. M., Higgins, S., McAuliffe, F. M., & Cotter, P. D. (2020). Shotgun sequencing of the vaginal microbiome reveals both a species and functional potential signature of preterm birth. Npj Biofilms and Microbiomes, 6(1), 50. https://doi.org/10.1038/s41522-020-00162-8
- Fettweis, J. M., Brooks, J. P., Serrano, M. G., Sheth, N. U., Girerd, P. H., Edwards, D. J., Strauss, J. F., Consortium, T. V. M., Jefferson, K. K., & Buck, G. A. (2014). Differences in vaginal microbiome in African American women versus women of European ancestry. Microbiology (Reading, England), 160(Pt 10), 2272–2282. https://doi.org/10.1099/mic.0.081034-0
- Fettweis, J. M., Serrano, M. G., Brooks, J. P., Edwards, D. J., Girerd, P. H., Parikh, H. I., Huang, B., Arodz, T. J., Edupuganti, L., Glascock, A. L., Xu, J., Jimenez, N. R., Vivadelli, S. C., Fong, S. S., Sheth, N. U., Jean, S., Lee, V., Bokhari, Y. A., Lara, A. M., … Buck, G. A. (2019). The vaginal microbiome and preterm birth. Nature Medicine, 25(6), 1012–1021. https://doi.org/10.1038/s41591-019-0450-2
- Foxman B. (2014). Urinary tract infection syndromes: occurrence, recurrence, bacteriology, risk factors, and disease burden. Infectious disease clinics of North America, 28(1), 1–13. https://doi.org/10.1016/j.idc.2013.09.003
- France, M. T., Ma, B., Gajer, P., Brown, S., Humphrys, M. S., Holm, J. B., Waetjen, L. E., Brotman, R. M., & Ravel, J. (2020). VALENCIA: a nearest centroid classification method for vaginal microbial communities based on composition. Microbiome, 8(1), 166. https://doi.org/10.1186/s40168-020-00934-6
- Gajer, P., Brotman, R. M., Bai, G., Sakamoto, J., Schütte, U. M., Zhong, X., Koenig, S. S., Fu, L., Ma, Z. S., Zhou, X., Abdo, Z., Forney, L. J., & Ravel, J. (2012). Temporal dynamics of the human vaginal microbiota. Science translational medicine, 4(132), 132ra52. https://doi.org/10.1126/scitranslmed.3003605
- Goldacre, M. J., Watt, B., Loudon, N., Milne, L. J., Loudon, J. D., & Vessey, M. P. (1979). Vaginal microbial flora in normal young women. British Medical Journal, 1(6176), 1450. https://doi.org/10.1136/bmj.1.6176.1450
- Greenbaum, S., Greenbaum, G., Moran-Gilad, J., & Weintraub, A. Y. (2019). Ecological dynamics of the vaginal microbiome in relation to health and disease. American Journal of Obstetrics and Gynecology, 220(4), 324–335. https://doi.org/https://doi.org/10.1016/j.ajog.2018.11.1089
- Gupta, P., Singh, M. P., & Goyal, K. (2020). Diversity of Vaginal Microbiome in Pregnancy: Deciphering the Obscurity. Frontiers in Public Health, 8, 326. https://www.frontiersin.org/article/10.3389/fpubh.2020.00326
- Gupta, S., Kakkar, V., & Bhushan, I. (2019). Crosstalk between Vaginal Microbiome and Female Health: A review. Microbial Pathogenesis, 136, 103696. https://doi.org/10.1016/j.micpath.2019.103696
- Happel, A. U., Varsani, A., Balle, C., Passmore, J. A., & Jaspan, H. (2020). The Vaginal Virome-Balancing Female Genital Tract Bacteriome, Mucosal Immunity, and Sexual and Reproductive Health Outcomes?. Viruses, 12(8), 832. https://doi.org/10.3390/v12080832
- Hemmerling, A., Harrison, W., Schroeder, A., Park, J., Korn, A., Shiboski, S., Foster-Rosales, A., & Cohen, C. R. (2010). Phase 2a Study Assessing Colonization Efficiency, Safety, and Acceptability of Lactobacillus crispatus CTV-05 in Women With Bacterial Vaginosis. Sexually Transmitted Diseases, 37(12). https://journals.lww.com/stdjournal/Fulltext/2010/12000/Phase_2a_Study_Assessing_Colonization_Efficiency,.3.aspx
- Hillebrand, L., Harmanli, O. H., Whiteman, V., & Khandelwal, M. (2002). Urinary tract infections in pregnant women with bacterial vaginosis. American journal of obstetrics and gynecology, 186(5), 916–917. https://doi.org/10.1067/mob.2002.123987
- Hooton, T. M. (2012). Uncomplicated Urinary Tract Infection. New England Journal of Medicine, 366(11), 1028–1037. https://doi.org/10.1056/NEJMcp1104429
- Hyman, R. W., Fukushima, M., Jiang, H., Fung, E., Rand, L., Johnson, B., Vo, K. C., Caughey, A. B., Hilton, J. F., Davis, R. W., & Giudice, L. C. (2014). Diversity of the Vaginal Microbiome Correlates With Preterm Birth. Reproductive Sciences, 21(1), 32–40. https://doi.org/10.1177/1933719113488838
- Jang, S. J., Lee, K., Kwon, B., You, H. J., & Ko, G. (2019). Vaginal lactobacilli inhibit growth and hyphae formation of Candida albicans. Scientific Reports, 9(1), 8121. https://doi.org/10.1038/s41598-019-44579-4
- Kim, J.-M., & Park, Y. J. (2017). Probiotics in the Prevention and Treatment of Postmenopausal Vaginal Infections: Review Article. Jmm, 23(3), 139–145. https://doi.org/10.6118/jmm.2017.23.3.139
- Kolter, J., & Henneke, P. (2017). Codevelopment of Microbiota and Innate Immunity and the Risk for Group B Streptococcal Disease. Frontiers in Immunology, 8, 1497. https://www.frontiersin.org/article/10.3389/fimmu.2017.01497
- Lagenaur, L. A., Hemmerling, A., Chiu, C., Miller, S., Lee, P. P., Cohen, C. R., & Parks, T. P. (2021). Connecting the Dots: Translating the Vaginal Microbiome Into a Drug. The Journal of Infectious Diseases, 223(Supplement_3), S296–S306. https://doi.org/10.1093/infdis/jiaa676
- Lai, S. K., Hida, K., Shukair, S., Wang, Y. Y., Figueiredo, A., Cone, R., Hope, T. J., & Hanes, J. (2009). Human immunodeficiency virus type 1 is trapped by acidic but not by neutralized human cervicovaginal mucus. Journal of virology, 83(21), 11196–11200. https://doi.org/10.1128/JVI.01899-08
- Lee, J. B. L., & Neild, G. H. (2007). Urinary tract infection. Medicine, 35(8), 423–428. https://doi.org/10.1016/j.mpmed.2007.05.009
- Lewis, A. L., & Gilbert, N. M. (2020). Roles of the vagina and the vaginal microbiota in urinary tract infection: evidence from clinical correlations and experimental models. GMS Infectious Diseases, 8, Doc02–Doc02. https://doi.org/10.3205/id000046
- Liebenberg, L. J. P., Masson, L., Arnold, K. B., Mckinnon, L. R., Werner, L., Proctor, E., Archary, D., Mansoor, L. E., Lauffenburger, D. A., Abdool Karim, Q., Abdool Karim, S. S., & Passmore, J.-A. S. (2017). Genital-Systemic Chemokine Gradients and the Risk of HIV Acquisition in Women. Journal of Acquired Immune Deficiency Syndromes (1999), 74(3), 318–325. https://doi.org/10.1097/QAI.0000000000001218
- Ma, B., Forney, L. J., & Ravel, J. (2012). Vaginal Microbiome: Rethinking Health and Disease. Annual Review of Microbiology, 66(1), 371–389. https://doi.org/10.1146/annurev-micro-092611-150157
- Mancabelli, L., Tarracchini, C., Milani, C., Lugli, G. A., Fontana, F., Turroni, F., van Sinderen, D., & Ventura, M. (2021). Vaginotypes of the human vaginal microbiome. Environmental Microbiology, 23(3), 1780–1792. https://doi.org/https://doi.org/10.1111/1462-2920.15441
- Margolis, E., & Fredricks, D. N. (2015). Chapter 83 – Bacterial Vaginosis-Associated Bacteria. In Y.-W. Tang, M. Sussman, D. Liu, I. Poxton, & J. Schwartzman (Eds.), Molecular Medical Microbiology (Second Edition) (pp. 1487–1496). Academic Press. https://doi.org/10.1016/B978-0-12-397169-2.00083-4
- Marrazzo, J. M., Fiedler, T. L., Srinivasan, S., Thomas, K. K., Liu, C., Ko, D., Xie, H., Saracino, M., & Fredricks, D. N. (2012). Extravaginal Reservoirs of Vaginal Bacteria as Risk Factors for Incident Bacterial Vaginosis. The Journal of Infectious Diseases, 205(10), 1580–1588. https://doi.org/10.1093/infdis/jis242
- Martin, D. H., & Marrazzo, J. M. (2016). The Vaginal Microbiome: Current Understanding and Future Directions. The Journal of Infectious Diseases, 214(suppl_1), S36–S41. https://doi.org/10.1093/infdis/jiw184
- Martin, H. L., Richardson, B. A., Nyange, P. M., Lavreys, L., Hillier, S. L., Chohan, B., Mandaliya, K., Ndinya-Achola, J. O., Bwayo, J., & Kreiss, J. (1999). Vaginal lactobacilli, microbial flora, and risk of human immunodeficiency virus type 1 and sexually transmitted disease acquisition. The Journal of infectious diseases, 180(6), 1863–1868. https://doi.org/10.1086/315127
- Miller, C. J., & Shattock, R. J. (2003). Target cells in vaginal HIV transmission. Microbes and Infection, 5(1), 59–67. https://doi.org/10.1016/S1286-4579(02)00056-4
- Mitchell, H. (2004). Vaginal discharge—causes, diagnosis, and treatment. BMJ, 328(7451), 1306. https://doi.org/10.1136/bmj.328.7451.1306
- Molenaar, M. C., Singer, M., & Ouburg, S. (2018). The two-sided role of the vaginal microbiome in Chlamydia trachomatis and Mycoplasma genitalium pathogenesis. Journal of reproductive immunology, 130, 11–17. https://doi.org/10.1016/j.jri.2018.08.006
- Murphy, K., & Mitchell, C. M. (2016). The Interplay of Host Immunity, Environment and the Risk of Bacterial Vaginosis and Associated Reproductive Health Outcomes. The Journal of Infectious Diseases, 214(suppl_1), S29–S35. https://doi.org/10.1093/infdis/jiw140
- Neggers, Y. H., Nansel, T. R., Andrews, W. W., Schwebke, J. R., Yu, K., Goldenberg, R. L., & Klebanoff, M. A. (2007). Dietary Intake of Selected Nutrients Affects Bacterial Vaginosis in Women. The Journal of Nutrition, 137(9), 2128–2133. https://doi.org/10.1093/jn/137.9.2128
- Nicolle, L. E., Harding, G. K., Preiksaitis, J., & Ronald, A. R. (1982). The association of urinary tract infection with sexual intercourse. The Journal of infectious diseases, 146(5), 579–583. https://doi.org/10.1093/infdis/146.5.579
- Nugent, R. P., Krohn, M. A., & Hillier, S. L. (1991). Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. Journal of Clinical Microbiology, 29(2), 297–301. https://doi.org/10.1128/jcm.29.2.297-301.1991
- Oduyebo, O. O., Anorlu, R. I., & Ogunsola, F. T. (2009). The effects of antimicrobial therapy on bacterial vaginosis in non-pregnant women. The Cochrane database of systematic reviews, (3), CD006055. https://doi.org/10.1002/14651858.CD006055.pub2
- Oerlemans, E. F. M., Bellen, G., Claes, I., Henkens, T., Allonsius, C. N., Wittouck, S., van den Broek, M. F. L., Wuyts, S., Kiekens, F., Donders, G. G. G., & Lebeer, S. (2020). Impact of a lactobacilli-containing gel on vulvovaginal candidosis and the vaginal microbiome. Scientific Reports, 10(1), 7976. https://doi.org/10.1038/s41598-020-64705-x
- Onderdonk, A. B., Delaney, M. L., & Fichorova, R. N. (2016). The Human Microbiome during Bacterial Vaginosis. Clinical Microbiology Reviews, 29(2), 223–238. https://doi.org/10.1128/CMR.00075-15
- Peipert, J. F., Lapane, K. L., Allsworth, J. E., Redding, C. A., Blume, J. D., & Stein, M. D. (2008). Bacterial vaginosis, race, and sexually transmitted infections: does race modify the association?. Sexually transmitted diseases, 35(4), 363–367. https://doi.org/10.1097/OLQ.0b013e31815e4179
- Peters, B. M., Yano, J., Noverr, M. C., & Fidel Jr, P. L. (2014). Candida Vaginitis: When Opportunism Knocks, the Host Responds. PLOS Pathogens, 10(4), e1003965-. https://doi.org/10.1371/journal.ppat.1003965
- Petrova, M. I., Imholz, N. C. E., Verhoeven, T. L. A., Balzarini, J., van Damme, E. J. M., Schols, D., Vanderleyden, J., & Lebeer, S. (2016). Lectin-Like Molecules of Lactobacillus rhamnosus GG Inhibit Pathogenic Escherichia coli and Salmonella Biofilm Formation. PLOS ONE, 11(8), e0161337-. https://doi.org/10.1371/journal.pone.0161337
- Phares, C. R., Lynfield, R., Farley, M. M., Mohle-Boetani, J., Harrison, L. H., Petit, S., Craig, A. S., Schaffner, W., Zansky, S. M., Gershman, K., Stefonek, K. R., Albanese, B. A., Zell, E. R., Schuchat, A., & Schrag, S. J. (2008). Epidemiology of Invasive Group B Streptococcal Disease in the United States, 1999-2005. JAMA, 299(17), 2056–2065. https://doi.org/10.1001/jama.299.17.2056
- Pino, A., Bartolo, E., Caggia, C., Cianci, A., & Randazzo, C. L. (2019). Detection of vaginal lactobacilli as probiotic candidates. Scientific Reports, 9(1), 3355. https://doi.org/10.1038/s41598-019-40304-3
- Prais, D., Straussberg, R., Avitzur, Y., Nussinovitch, M., Harel, L., & Amir, J. (2003). Bacterial susceptibility to oral antibiotics in community acquired urinary tract infection. Archives of Disease in Childhood, 88(3), 215. https://doi.org/10.1136/adc.88.3.215
- Prince, A. L., Chu, D. M., Seferovic, M. D., Antony, K. M., Ma, J., & Aagaard, K. M. (2015). The Perinatal Microbiome and Pregnancy: Moving Beyond the Vaginal Microbiome. Cold Spring Harbor Perspectives in Medicine, 5(6). https://doi.org/10.1101/cshperspect.a023051
- Quin, C., Vollman, D. M., Ghosh, S., Haskey, N., Estaki, M., Pither, J., Barnett, J. A., Jay, M. N., Birnie, B. W., & Gibson, D. L. (2020). Fish oil supplementation reduces maternal defensive inflammation and predicts a gut bacteriome with reduced immune priming capacity in infants. The ISME Journal, 14(8), 2090–2104. https://doi.org/10.1038/s41396-020-0672-9
- Rautava, S., Luoto, R., Salminen, S., & Isolauri, E. (2012). Microbial contact during pregnancy, intestinal colonization and human disease. Nature Reviews Gastroenterology & Hepatology, 9(10), 565–576. https://doi.org/10.1038/nrgastro.2012.144
- Ravel, J., Gajer, P., Abdo, Z., Schneider, G. M., Koenig, S. S. K., McCulle, S. L., Karlebach, S., Gorle, R., Russell, J., Tacket, C. O., Brotman, R. M., Davis, C. C., Ault, K., Peralta, L., & Forney, L. J. (2011). Vaginal microbiome of reproductive-age women. Proceedings of the National Academy of Sciences, 108(Supplement 1), 4680. https://doi.org/10.1073/pnas.1002611107
- Raz, R., & Stamm, W. E. (1993). A controlled trial of intravaginal estriol in postmenopausal women with recurrent urinary tract infections. The New England journal of medicine, 329(11), 753–756. https://doi.org/10.1056/NEJM199309093291102
- Schwebke, J. R. (2000). Asymptomatic bacterial vaginosis: Response to therapy. American Journal of Obstetrics and Gynecology, 183(6), 1434–1439. https://doi.org/https://doi.org/10.1067/mob.2000.107735
- Sewankambo, N., Gray, R. H., Wawer, M. J., Paxton, L., McNaim, D., Wabwire-Mangen, F., Serwadda, D., Li, C., Kiwanuka, N., Hillier, S. L., Rabe, L., Gaydos, C. A., Quinn, T. C., & Konde-Lule, J. (1997). HIV-1 infection associated with abnormal vaginal flora morphology and bacterial vaginosis. Lancet (London, England), 350(9077), 546–550. https://doi.org/10.1016/s0140-6736(97)01063-5
- Sheerin, N. S. (2011). Urinary tract infection. Medicine, 39(7), 384–389. https://doi.org/10.1016/j.mpmed.2011.04.003
- Siqueira, J. D., Curty, G., Xutao, D., Hofer, C. B., Machado, E. S., Seuánez, H. N., Soares, M. A., Delwart, & E., Soares, E. A. (2019). Composite Analysis of the Virome and Bacteriome of HIV/HPV Co-Infected Women Reveals Proxies for Immunodeficiency. Viruses, 11(5):422. https://doi.org/10.3390/v11050422
- Stapleton A. E. (2016). The Vaginal Microbiota and Urinary Tract Infection. Microbiology spectrum, 4(6), 10.1128/microbiolspec.UTI-0025-2016. https://doi.org/10.1128/microbiolspec.UTI-0025-2016
- Stapleton, A. E., Au-Yeung, M., Hooton, T. M., Fredricks, D. N., Roberts, P. L., Czaja, C. A., Yarova-Yarovaya, Y., Fiedler, T., Cox, M., & Stamm, W. E. (2011). Randomized, placebo-controlled phase 2 trial of a Lactobacillus crispatus probiotic given intravaginally for prevention of recurrent urinary tract infection. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America, 52(10), 1212–1217. https://doi.org/10.1093/cid/cir183
- Sumati, A. H., & Saritha, N. K. (2009). Association of urinary tract infection in women with bacterial vaginosis. Journal of global infectious diseases, 1(2), 151–152. https://doi.org/10.4103/0974-777X.56254
- Swidsinski, A., Mendling, W., Loening-Baucke, V., Swidsinski, S., Dörffel, Y., Scholze, J., Lochs, H., & Verstraelen, H. (2008). An adherent Gardnerella vaginalis biofilm persists on the vaginal epithelium after standard therapy with oral metronidazole. American Journal of Obstetrics and Gynecology, 198(1), 97.e1-97.e6. https://doi.org/10.1016/j.ajog.2007.06.039
- Ta, L. D. H., Chan, J. C. Y., Yap, G. C., Purbojati, R. W., Drautz-Moses, D. I., Koh, Y. M., Tay, C. J. X., Huang, C.-H., Kioh, D. Y. Q., Woon, J. Y., Tham, E. H., Loo, E. X. L., Shek, L. P. C., Karnani, N., Goh, A., van Bever, H. P. S., Teoh, O. H., Chan, Y. H., Lay, C., … Lee, B. W. (2020). A compromised developmental trajectory of the infant gut microbiome and metabolome in atopic eczema. Gut Microbes, 12(1), 1801964. https://doi.org/10.1080/19490976.2020.1801964
- Tachedjian, G., Aldunate, M., Bradshaw, C. S., & Cone, R. A. (2017). The role of lactic acid production by probiotic Lactobacillus species in vaginal health. Research in Microbiology, 168(9), 782–792. https://doi.org/10.1016/j.resmic.2017.04.001
- Taha, T. E., Hoover, D. R., Dallabetta, G. A., Kumwenda, N. I., Mtimavalye, L. A. R., Yang, L.-P., Liomba, G. N., Broadhead, R. L., Chiphangwi, J. D., & Miotti, P. G. (1998). Bacterial vaginosis and disturbances of vaginal flora: association with increased acquisition of HIV. AIDS, 12(13). https://journals.lww.com/aidsonline/Fulltext/1998/13000/Bacterial_vaginosis_and_disturbances_of_vaginal.19.aspx
- Tan, C. W., & Chlebicki, M. P. (2016). Urinary tract infections in adults. Singapore Medical Journal, 57(9), 485–490. https://doi.org/10.11622/smedj.2016153
- Tazi, A., Plainvert, C., Anselem, O., Ballon, M., Marcou, V., Seco, A., el Alaoui, F., Joubrel, C., el Helali, N., Falloukh, E., Frigo, A., Raymond, J., Trieu-Cuot, P., Branger, C., le Monnier, A., Azria, E., Ancel, P.-Y., Jarreau, P. H., Mandelbrot, L., … Poyart, C. (2019). Risk Factors for Infant Colonization by Hypervirulent CC17 Group B Streptococcus: Toward the Understanding of Late-onset Disease. Clinical Infectious Diseases, 69(10), 1740–1748. https://doi.org/10.1093/cid/ciz033
- Torcia, M. G. (2019). Interplay among Vaginal Microbiome, Immune Response and Sexually Transmitted Viral Infections. International Journal of Molecular Sciences, 20(2):266. https://doi.org/10.3390/ijms20020266
- van de Wijgert, J., & Verwijs, M. C. (2020). Lactobacilli-containing vaginal probiotics to cure or prevent bacterial or fungal vaginal dysbiosis: a systematic review and recommendations for future trial designs. BJOG: An International Journal of Obstetrics & Gynaecology, 127(2), 287–299. https://doi.org/10.1111/1471-0528.15870
- Verstraelen, H., Verhelst, R., Vaneechoutte, M., & Temmerman, M. (2010). The epidemiology of bacterial vaginosis in relation to sexual behaviour. BMC Infectious Diseases, 10(1), 81. https://doi.org/10.1186/1471-2334-10-81
- Wiesenfeld, H. C., Hillier, S. L., Krohn, M. A., Landers, D. v, & Sweet, R. L. (2003). Bacterial Vaginosis Is a Strong Predictor of Neisseria gonorrhoeae and Chlamydia trachomatis Infection. Clinical Infectious Diseases, 36(5), 663–668. https://doi.org/10.1086/367658
- Wylie, K. M., Wylie, T. N., Cahill, A. G., Macones, G. A., Tuuli, M. G., & Stout, M. J. (2018). The vaginal eukaryotic DNA virome and preterm birth. American Journal of Obstetrics and Gynecology, 219(2), 189.e1-189.e12. https://doi.org/10.1016/j.ajog.2018.04.048
- Xu, J., Bian, G., Zheng, M., Lu, G., Chan, W.-Y., Li, W., Yang, K., Chen, Z.-J., & Du, Y. (2020). Fertility factors affect the vaginal microbiome in women of reproductive age. American Journal of Reproductive Immunology, 83(4), e13220. https://doi.org/10.1111/aji.13220
- Zabor, E. C., Klebanoff, M., Yu, K., Zhang, J., Nansel, T., Andrews, W., Schwebke, J., & Jeffcoat, M. (2010). Association between periodontal disease, bacterial vaginosis, and sexual risk behaviours. Journal of Clinical Periodontology, 37(10), 888–893. https://doi.org/10.1111/j.1600-051X.2010.01593.x
- Zaleznik, D. F., Rench, M. A., Hillier, S., Krohn, M. A., Platt, R., Lee, M.-L. T., Flores, A. E., Ferrieri, P., & Baker, C. J. (2000). Invasive Disease Due to Group B Streptococcus in Pregnant Women and Neonates from Diverse Population Groups. Clinical Infectious Diseases, 30(2), 276–281. https://doi.org/10.1086/313665
- Zapata, H. J., & Quagliarello, V. J. (2015). The Microbiota and Microbiome in Aging: Potential Implications in Health and Age-Related Diseases. Journal of the American Geriatrics Society, 63(4), 776–781. https://doi.org/10.1111/jgs.13310
- Zozaya, M., Ferris, M. J., Siren, J. D., Lillis, R., Myers, L., Nsuami, M. J., Eren, A. M., Brown, J., Taylor, C. M., & Martin, D. H. (2016). Bacterial communities in penile skin, male urethra, and vaginas of heterosexual couples with and without bacterial vaginosis. Microbiome, 4(1), 16. https://doi.org/10.1186/s40168-016-0161-6

